Respiratory Medicine
Lung Development
Michael Finelli, RRT
The material presented here was first published in the Residents’ Handbook of Neonatology, 3rd edition, and is reproduced here with permission from PMPH USA, Ltd. of New Haven, Connecticut and Cary, North Carolina.
Lungs develop from a ventral diverticulum, from caudal foregut at 3 weeks embryonic life. In five stages, the lung bud branch and penetrates the mesenchyme, and specific abnormalities occur by stage of development. At any stage, hypoplastic anomalies can result from compression (inadequate uterine space), or of a reduction in either fetal breathing movements or amniotic fluid volume. The fetal lung is an excretory organ; producing 2 – 5 ml/kg/hr of lung fluid. The lung fluid present in a term infant immediately prior to delivery amounts to 30 ml/kg. The mechanism for cessation of lung fluid production prior to delivery involves both steroid induced maturation and hormonal signalling.
| Stage of Development | Gestational age | Associated abnormality |
|---|---|---|
| Embryonic | 3-6 weeks |
|
| Pseudoglandular | 6-16 weeks |
|
| Canalicular | 16-26 weeks | |
| Terminal sac | 26-36 weeks | Pulmonary hypoplasia |
| Alveolar | 36 wks – infancy | Surfactant deficiency |
- Gaseous exchange is possible towards end of the canalicular phase. By 20 – 22 weeks both type I and type II pneumocytes are present; by 24 weeks surfactant is present in minimal concentration.
- An important component of extra-uterine adaptation is for the lung to convert from an excretory organ to a gaseous exchange organ.
- Goal of respiratory support in newborn infants, is to provide adequate oxygenation and carbon dioxide removal whilst avoiding hyperoxaemia and hypocapnia.
Oxygen transport
Energy production requires supply of oxygen to the mitochondria, producing high energy phosphate bonds (e.g. ATP) for oxidative phosphorylation.
Haemoglobin in red cells reversibly binds oxygen, in a sigmoid fashion
(Diagram… Haemoglobin-oxygen dissociation curve).
Legend:
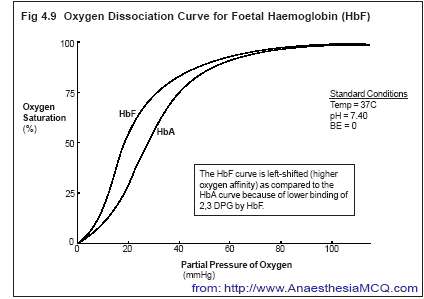
This diagram shows the so-called sigmoid pattern – a slow decline in oxygen saturation from 100 to 75% saturation with falling oxygen partial pressures – and thereafter a sudden decline on the steep portion. Shown here are both adult and fetal hemoglobin, showing that HbF is ‘more avid’ for oxygen at any given partial pressure.
Each molecule of haemoglobin binding 4 molecules of O2. Factors affecting haemoglobin’s affinity for oxygen include:
- Amount of fetal haemoglobin (HbF);
- Amount of 2,3-diphosphoglycerate (DPG);
- pH;
- PCO2;
- Temperature.
Oxygen Delivery:
Oxygen delivery is determined by haemoglobin levels, its Hb-oxygen saturation, cardiac output, and oxygen unloading from haemoglobin to the tissues.
Adequate Oxygenation:
is a balance between the rate of oxygen delivery to the tissues and their rate of oxygen consumption.
- Generally, O2 demands of preterm infants can be met by maintaining PaO2 levels just above 50 mm Hg or SaO2 levels just above 88%.
- Under normal circumstances, oxygen consumption for a neonate is approximately 6 ml/kg/min (from a total of 24 ml/kg/min delivered to the systemic circulation).
- Therefore, approximately 25% of the oxygen is removed from the blood by the time it returns to the heart.
Hypoxia and hypoxaemia
Definitions:
- Hypoxia – inadequate oxygen delivery for the tissue needs.
- Hypoxaemia – low arterial blood oxygen (low PaO2).
Frequently these occur together but they are not synonymous.
Tissue hypoxia can occur even in the presence of adequate PaO2 (e.g. poor cardiac output, severe anaemia, methaemoglobinaemia).
Presence of hypoxia indicates tissue oxygen deprivation, and is indicated by lactic acidosis and is important to follow serial serum lactates.
Hyperoxaemia
- High levels of oxygen are toxic to a number of organs in newborns.
- The safe upper limits are still uncertain, although trials are now starting to try to define this in infants <30 weeks gestation.
- Currently we aim for PaO2 50-70 mmHg and saturations 90-95 for all infants
- Pragmatically it can be defined as a function of gestational age:-
Swings in oxygenation are to be avoided.
Oxygen Index
Useful measure of the intensity of respiratory support required to maintain adequate oxygenation. Most often used as guide to action in Persistent Pulmonary Hypertension Newborn (PPHN). However, the OI can be slightly exaggerated if the infant is on HFOV since the MAP is often higher than what may be required on conventional ventilation
Oxygenation index (OI) = (MAP x FiO2 x 100)
Postductal PaO2
OI = oxygen index
MAP = mean airway pressure (cm H2O)
FiO2 = inhaled oxygen
PaO2 = postductal PaO2
An OI > 15 signifies severe respiratory compromise;
Monitoring Oxygen
Well controlled O2 therapy reduces the risk of pulmonary vasoconstriction and retinopathy of prematurity.
Because of the sigmoid dissociation curve, and the effects of metabolism, saturations are only a guide to the real oxygenation of the infant; therefore, it is advisable to check true PaO2 regularly. Blood gas measurement from an in-dwelling arterial catheter is the “gold standard” of assessing adequacy of ventilation and oxygenation.
Indications for monitoring:
- Preterm < 35 weeks;
- Oxygen therapy or its recent discontinuation;
- Respiratory distress;
- Severe cardiovascular disease;
- Risk of apnoeic episodes;
- Suspected cyanosis.
Use both low and high setting alarms to avoid hyperoxaemia as well as hypoxaemia.
Indications for arterial catheter:
Unstable patient, requirement for frequent blood gas monitoring
Mainstay of monitoring in NICU; requires no calibration. Utilises the principle that saturated haemoglobin is a different colour than desaturated haemoglobin. Light emission into the skin is reflected and sensed; since tissue light absorption differs from both the amount of blood in the tissue, and the relative amounts of oxygenated and deoxygenated haemoglobin. In order of importance, motion, poor skin perfusion, ambient light, skin pigmentation, may reduce saturations and interfere with accuracy. Note that dyshaemoglobinaemia causes inaccurately high SpO2 readings.
Used in the neonatal population to prevent prolonged hypoxaemia and hyperoxaemia: both a high and low limit alarm should be set. Most manufacturers claim confidence intervals of ± 4% for readings above 70%; below 70%, precision is substantially less.
- Pay attention to wide fluctuations – seek reason(s). i.e. patent ductus arteriosus
Current practice based on available evidence includes maintaining saturations between 90-95 % if the infant is requiring supplemental oxygen, recognizing that there is also significant sequelae with hyperoxia and unnecessary administration of supplemental oxygen.

Pre- and post-ductal saturations
Right to left shunt can be suspected using two pulse oximeters, one placed on the right upper limb and one on the toe or foot, – when present the lower limb reads significantly lower saturations than that placed pre-ductally.
Consider significant difference as > 10%
A clinician must keep in mind that shunting could be occurring intrapulmonary and may not be revealed using pre and post ductal saturation differences.
Transcutaneous monitoring
Transcutaneous oxygen and carbon dioxide electrodes allow continuous indirect estimation of PaCO2 and PaO2. Ease of use means pulse oximetry has largely supplanted the use of transcutaneous monitoring in the NICU.
Hyperoxia test
To differentiate congenital, cyanotic heart disease from pulmonary disease, the infant is administered 100% oxygen for 5-10 minutes. The PaO2 obtained is compared to the PaO2 obtained previously: an increase in PaO2 to >150 mmHg excludes most types of cyanotic heart disease and suggests a pulmonary origin of the disease.2 The accuracy of this test is increased when concurrent end expiratory pressure is provided by CPAP.
Carbon Dioxide
Produced by the tissues as product of aerobic respiration, returned to the lungs by the blood where it is exhaled. CO2 is transported in the blood as bicarbonate ion, on haemoglobin and as dissolved gas, or as carbonic acid, in the plasma.
Monitoring Carbon Dioxide
The PaCO2 level from an indwelling arterial catheter provides the most accurate method of determining adequacy of alveolar ventilation.
Two other methods are used with different frequency in different units:
End-tidal
1. CO2 estimation (capnography).
It is relatively in-expensive, portable, non-invasive and easy to use. Alveolar CO2 approximates PaCO2 therefore a sample of end-tidal CO2 gives a good estimate of PaCO2, in some patient populations The value becomes difficult to interpret and often much lower than actual if the patient is breathing at RR >60 as there is not enough time to accurately measure the exhaled CO2. Is can be used to corroborate that the endotracheal tube is in the trachea following intubation. It does not however ensure optimal position of the ETT. Most often disposable calorimetric filter devices are used for this purpose.
2. Transcutaneous CO2
Provides continuous, non-invasive monitoring. TcPCO2 is always greater than PCO2. Provides a fairly linear relationship between TcPCO2 and PaCO2. One must understand that these device do have limitations based on site selection and perfusion and should be correlated to an arterial blood gas to ensure proper trending can be established.
Permissive Hypercapnia Strategies in Mechanical Ventilation
The accent in ventilatory management has moved away from maintenance of normal blood gases.
Ventilatory strategies associated with hypocapnia (PaCO2 < 30 mm Hg) in the first 96 hours of life are associated with increased incidence of bronchopulmonary dysplasia and periventricular leukomalacia and neurodevelopmental delay.
Keeping the PaCO2 in the “normal range”, will result in ventilator induced lung injury (VILI), and is not advised. Instead permissive hypercapnia is usually adopted. Permissive hypercapnia is a proven “lung-protective strategy” in adult patients, but data for efficacy and safety are limited. Current evidence indicates that PaCO2 levels of 45 – 55 mm Hg are safe and well tolerated.4 This strategy relies on a determined CPAP approach to deflect intubation in many infants; and by a combination of decreasing respiratory rate, inspiratory pressures, or low tidal volumes – during mechanical ventilation.
Note, however, ventilating low volume lungs with small tidal volumes may result in a progressive loss of lung volume/atelectasis and surfactant dysfunction. Volume targets should be no lower than 4cc/kg when ventilating and infant with both left and right lungs.
Appropriate levels of positive end expiratory pressure (PEEP) must be used to ensure maintenance of this functional residual capacity (FRC).3
Respiratory Assistance
Goals are to:
- Maintain respiratory gas exchange;
- Promote cardiovascular stability;
- Lower work of breathing and metabolic rate;
- Minimise risk of complications
Oxygen
- either high flow or low flow nasal cannulae
- headbox although rare now will often cause cooling of the head and face
Non invasive respiratory support
- Continuous positive airway pressure (nCPAP)
- Nasal intermittent positive pressure ventilation (nCMV/NIV/NIPPV)
Invasive Respiratory Support
- Intermittent mandatory ventilation (IMV) rare and archaic
- Synchronised intermittent mandatory ventilation (SIMV) (pressure controlled or volume targeted)
- Patient-triggered ventilation (PTV) or assist/control ventilation (pressure controlled or volume targeted) ]
- High frequency
- High frequency oscillatory ventilation (HFOV)
- High frequency jet ventilation (HFJV)
Intubation
Indications:
- Resuscitation
- Severe respiratory failure
- Repeated apnoeas with bradycardia
- Intractable cardiovascular instability
- Airway abnormalities causing severe obstruction
- Lack of protective reflexes to protect and maintain airway (suck, gag, cough)
- Hypoventilation syndromes i.e. CCHS
Premedication
To be routinely use for elective and semi-elective intubations. Suggested medications
- Atropine 20 micrograms/kg IV optional, to be avoided if tachycardia exists
- Fentanyl 2-3 micrograms/kg IV slowly and flushed slowly to avoid chest wall rigidity If chest wall rigidity occurs quickly administer paralytic agent (Succinylcholine or Rocuronium)
- Succinylcholine 2 milligrams/kg IV
- Insert CPS statement link to include Rocuronium
Suggested Size of ETT guidelines for internal ETT diameter based on weight.
| ETT size Internal diameter (mm) | Weight (g) | Gestational age (weeks) |
|---|---|---|
| 2.5 | < 1000 | < 26 |
| 3.0 | 1000 – 2000 | 28 – 34 |
| 3.5 | 2000 – 3000 | 34 – 38 |
| 3.5 – 4.0 | > 3000 | > 38 |
Although there are individual differences, be careful not to use too large an ETT. This is associated with glottic oedema and fibrosis resulting in sub-glottic stenosis. It is important to recognize that using the weight criteria in patients who are SGA will often lead to insertion of a smaller than required airway and GA should be used instead in order to avoid large ETT leaks and inability to ventilate.
Surfactant
Routine part of clinical care:
- Type of surfactant
- Timing and size of initial dose
- New delivery methods
- Optimal ventilation strategy after treatment
- New indicators for treatment
- Next generation of surfactants.
Surfactant in RDS
Intubated infants with a diagnosis of RDS should receive surfactant therapy, if FiO2> 0.30 to maintain adequate oxygenation either on CPAP or following intubation. Trials of natural versus synthetic surfactants favour natural.5;6 Infants who are at significant risk of developing RDS (all infants <26 weeks and to those of 26 and 27 weeks with incomplete course of antenatal steroids). Delivery should be by rapid bolus for optimal distribution and physiological effects.7;8
Repeat Dosing
Infants with RDS with persisting, or recurrent, oxygen and ventilatory requirements should receive up to two further doses of surfactant: as early as two hours following the first dose or, more commonly, 4-6 hours after the initial dose. The clinician must be prepared to wean the PIPs and FiO2 rapidly or choose a volume targeted strategy that will wean for you. Following the administration of surfactant; one is often considering early extubation to CPAP.
A number of other diseases may benefit from the administration of surfactant therapy:, pulmonary haemorrhage, pneumonia and on some occasions meconium aspiration syndrome – the evidence for its use in these situations is not as strong as with RDS however, for severe cases, surfactant therapy may be considered as one aspect of the ongoing clinical care and is best utilized in level 3 centres that can provide HFOV, HFJV and inhaled nitric oxide as required.
nCPAP
Nasal continuous positive airway pressure, non-invasive positive respiratory support, reflects a continuous distending pressure to the airways in the spontaneously breathing infant throughout the respiratory cycle.9 Aim is to keep airways open and prevent collapse during expiration. Used in both preterm and term infants. Increasing interest in the era of attempting to reduce ventilator induced lung injury (VILI).
Large number of potential delivery systems for supplying nCPAP; fundamentally, however, the delivery of nCPAP requires three components:-
- Flow Generator – two types; constant and variable flow. Theoretical advantages of variable flow devices are not consistently supported by long-term clinically meaningful benefit. Pressure is dependent on flow used and is altered manually be the clinician. Variable leaks around the interface causes variable and often difficult to sustain CPAP levels.
- Airway Interface – a wide array of airway interfaces are available (table). Nasal CPAP is preferred because the infant can still be nursed and handled without interruption. Firm evidence for the comparative merits and risks of an individual interface is, on the whole, lacking, although short binasal prongs or masks are more effective in preterm infants at preventing reintubation compared to single nasal prongs8(NPTs).
- Positive Pressure System – 4 major pressure systems (table). At simplest, a fluid column will deliver bubble CPAP.
| Positive Pressure Systems |
|---|
|
| Physiologic Benefits of Continuous Positive Airway Pressure |
|---|
|
Indications for CPAP
Effective in supporting the recently extubated infant;15 for treating apnoea of prematurity;16 and increasingly as an alternative to intubation and ventilation in the treatment of RDS. Coupling CPAP with short duration intubation, early delivery of surfactant, for moderate to severe RDS, improves oxygenation and reduces the need for mechanical ventilation – INSURE technique (Intubation; Surfactant; rapid Extubation).13
Conditions where CPAP may not be useful include upper airway abnormalities, severe cardiovascular instability and intractable apnoeic episodes.
Optimal Pressure (Open up the lung and keep the lung open17)
No simple and reliable method to determine optimal pressure yet exists, this requires individualization. Care must be taken not to decrease the distending pressure below the closing pressure of the majority of the alveoli. Try to achieve the lowest possible pressure to maintain open alveoli without distension. Traditionally, pressures of 4 – 6 cm H2O have been used; other investigators have used pressures as high as 10-12 cm H2O. Many Canadian centers are currently using nCPAP pressures from 6 to 8 cm H2O and FiO2 >0.35 prior to deciding to intubate and give surfactant.
Insert AAP and European consensus statements/CPS if updated
We suggest individualization of the pressure to the infant’s requirements using the following indices
| Tailoring CPAP Pressure |
|---|
|
Starting pressure of 6-8 cm H2O. Using the above criteria (table) judicious increase the distending pressure in 1 cm H2O increments to a maximum of 10 cm H2O. Beware of gas trapping – O2 requirements increase as pressure increases. There are few clinical studies on this question, although older physiological studies would support this approach.18;19
Weaning CPAP
There are no controlled trials assessing the weaning of CPAP. One method is a 3 stage procedure:-
- Decrease the FiO2 to 0.30 whilst keeping SpO2 above 90%;
- Reduce pressure in 1 cm H2O increments to a pressure of 5 – 6 cm H2O;
- NB having an infant in higher fi02s in order to achieve a lower CPAP level may be counterproductive and cause oxidative stress and loss of lung volume.
- When the infant is considered ready for CPAP discontinuation. One must monitor the patient’s respiratory status and oxygen needs. For example if, removal from nCPAP causing increases oxygen requirements and increased work of breathing depicted by retractions, consider re initiating CPAP and discovering reason for failure. In the preterm population the emphasis post failure to come off CPAP should be focused on enhancing weight gain and calories and a period of 5-7 days should ensue before another trial off nCPAP.
- Cycling infants on and off nCPAP has not been proven to be a successful strategy and may result in greater length of stay Todd DA, Wright A, Broom M, et al. Methods of weaning preterm babies <30 weeks gestation off CPAP: a multicentre randomised controlled trial. Archs Dis Childh Fetal Neonatal Ed. 2012;97:F236–40.
Practical Considerations using CPAP
Should only be undertaken in units capable of performing rescue intubation, mechanical ventilation and. optimal gas humidification. nCPAP requires meticulous attention to the infant’s airway in terms of positioning and maintain patency and secretion clearance. Use the correct prong size that snugly fits the infant’s nares or mask that fits appropriately around nose. . Careful observation and care of the nose. Avoid excessive flexion or extension of the neck. The airway requires frequent suction to clear secretions, although how often this is required has not been studied. However, some NICUs suction the nasopharyngeal cavity 2 x per day and prn. Judicious use of vented gastric tube to decrease “CPAP belly/gastric distention”.
The use of CPAP requires constant observation of breathing patterns and standardized & rigorous training of respiratory therapists, physicians and nursing staff.
Potential complications of CPAP
Nasal complications include nasal pressure injury. There is conflicting information in the literature with regards to nCPAP and the occurrence of pneumothoraces as highlighted in both the COIN trial (which showed an increase PTX rate) and SUPPORT trial (which did not). All infants require careful monitoring for clinical deterioration. A sudden clinical deterioration although rare could indicate the presence of a pneumothorax and centers using nCPAP should be able to treat the pneumothorax.
Nasal Intermittent Positive Pressure Ventilation (NIPPV)
A newer strategy, that is currently only recommended in level 3 NICUs using NIPPV, via nasal prongs/mask with and without synchronization has been used as an alternative strategy of non-invasive respiratory support.
Synchronised NIPPV may provide superior short-term respiratory support for the recently extubated preterm infant.22 It is not yet known whether these short-term advantages translate into clinically meaningful long-term benefit however, it has been shown to increase extubation success in a recent Cochrane review http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD005384.pub2/pdf/abstract and another conducted by Haliday in 2004 link below
https://www.sciencedirect.com/science/article/pii/S1526054204900607
The link below is a great resource to support the information provided on CPAP: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4902733/pdf/nihms761408.pdf
Mechanical Ventilation
Ventilator Modes
- Classified according to which variables are set and controlled by the ventilator.
- Volume
- Pressure
- Flow
- Time
- Traditionally, neonatal ventilators were time cycled, constant flow generators and permitted control of inspiratory times, and pressure levels, which in turn delivered volume. However, modern day ventilators provide the clinician with a number of options to best suit the infant’s pathophysiology and needs.
Conventional Mechanical Ventilation
Assist / Control Ventilation or patient triggered ventilation
- Assist / Control ventilation (A/C) describes a mode of ventilation, in which the patient receives a full or mandatory breath on each effort.
- The rate set on the ventilator rate is the base rate and is the minimum rate the infant will receive.
- If the patient is breathing faster than the set respiratory rate, they receive a mandatory breath synchronized with each of their efforts – also known as an assisted breath
- Breaths may be supported with either a set pressure (using peak inspiratory pressure), or volume using a set target tidal volume With A/C ventilation, decreasing the set respiratory rate when the patient is breathing at a faster rate, will not change the patient’s minute ventilation, the infant continues to receive an assisted breath on each effort.
- If using A/C in combination with a volume targeted strategy, the peak inspiratory pressures will change based on the patients lung compliance, airway resistance and efforts to initiate the breath. The pressures will move up and down as required to sustain the targeted tidal volume which is currently the preferred strategy in many NICUs. The advantages of volume targeted ventilation is outlined in a recent Cochrane review conducted in 2017. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD003666.pub4/pdf/abstract
- If using A/C in a pressure delivery mode the user sets a PIP a Constant, pre-set pressure delivered through the inspiratory phase. The pressure is held for the duration of the set inspiratory time,
- The amount of flow will determine how quickly the pressure level is reached.
- Pressure ventilation results in a constant pressure displayed on a pressure-time waveform.
- The effective (difference between the peak inspiratory pressure (PIP) and the positive end expiratory pressure (PEEP)) pressure delivered results in a volume delivered. But this is often difficult to control and does not offer any automation in the face of resistance changes or compliance changes.
- In this mode, volume delivery changes with alterations in compliance and can lead to volutrauma if the lung compliance improves.
Synchronized Intermittent Mandatory Ventilation
- These mandatory breaths can be synchronized with the infant’s efforts –synchronized intermittent mandatory ventilation (SIMV).
- The base rate set on the machine is the maximum number of mechanical breaths that will be delivered.
- If the infant is breathing faster than the set ventilator, the infant receives the set number of breaths and the additional breaths are spontaneous or non-supported -essentially the patient is breathing from ETT CPAP.
Pressure Support
- Pressure support assists the infant to overcome the resistance of the endotracheal tube by assisting the patient’s spontaneous effort to a preset inspiratory pressure level.
- It basically gives the patient a small pressure “boost” above PEEP delivered down the airway to overcome the resistance. The patient is required to inflate their lungs beyond the boost as they wish. The ventilator will only add pressure with each patient effort, and again, the patient controls the respiratory rate, inspiratory time and volume delivered
- This option can be added to SIMV and help support the patients spontaneous breaths in terms of overcoming airway resistance.
- In SIMV+PS the support levels can be very different between mechanical and spontaneous breaths and the patient has varying control. The mandatory breaths are often limited by time (time-cycled) and in pressure support the transition from inspiration to expiration is determined by a decay in flow (flow cycled).
Ventilatory Parameters:
Inspiratory Times (Ti):
- Neonates breathe at a fast rate and have trouble staying synchronized if the inspiratory time is too long as you are trying to force them to do a breath hold. Conversely, setting the inspiratory time low will result in a lower MAP and may decrease oxygenation.
Clinical determination of Ti can be made by careful assessment of ventilator flow time
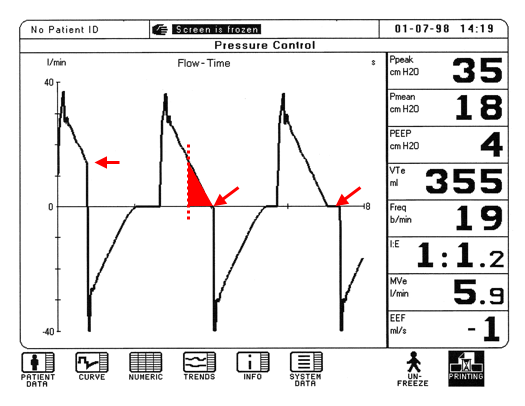
The diagram shows flow on the y-axis, by time on the x-axis.
For each breath, the area under the waveform represents delivered volume.
In the first breath, a very short inspiratory time and pressures cannot equilibrate – the end of the breath terminates abruptly. The area under the inspiratory flow curve is the inspired tidal volume which will now be decreased. The infant will often try to compensate for this by becoming tachypneic to manage the drop in their minute ventilation.
In the second breath, there is enough time to equilibrate the pressure, and additional volume is delivered (represented by the red triangle). This is referred to smooth transition to baseline.
In the third breath, increasing the inspiratory time further as in the third waveform shows no additional delivery of volume and results in a breath hold. Patients who are spontaneously breathing often resist breath holds and may begin to fight the ventilator and become asynchronous.
Mean Airway Pressure (MAP):
- Mean airway pressure is the area under a pressure-time waveform. All modern day ventilators display this calculated value. The ventilator parameters which affect MAP include inspiratory time (It), expiratory time (Et), PIP, PEEP and either the slope or rise time of the flow pattern. In a constant flow ventilator:
MAP = (Ti · PIP) + (Te · PEEP)
Ti + Te
- Following and trending the MAP is an easy way to track how the patient’s lung condition is changing. Worsening lung condition is often associated with an escalation in ventilator settings resulting in a higher calculated MAP.
Sensitivity
- Ventilators capable of synchronizing ventilation with the patient’s effort use either pressure or flow criteria to define ‘patient effort’. If the sensitivity criteria are met, the ventilator responds with an assisted or supported breath. Therefore sensitivity must be set appropriately to ensure the patient can access and interact with the respiratory support provided.
- Leak around the ETT, flow and / or pressure drop, may cause the ventilator to cycle the breath prematurely and this could lead to “auto triggering .
- Modern day ventilators often have algorithms built in to automatically adjust criteria in the face of changing ETT leaks this is known as leak adaptation.
| HFOV | |
|---|---|
| High Frequency Oscillation Ventilation(VG may be added) |
HFOV works by providing a consistent distending pressure known as mean airway pressure (MAP) to keep the lungs open and facilitate in oxygenation. HFO is powered by a forward and backward motion of a motorized piston diaphragm which generates an oscillation of waves known as amplitude at a very high frequency (measured in Hz where 1 HZ=60 br/min) to facilitate ventilation. The tidal volumes generated by HFOV are very small (≤ dead space volume). This is the only type of ventilation with ACTIVE EXHALATION which is achieved when the piston is in the backward position. The VN500 allows VG to be added to HFO. With every oscillated “breath”, the vent targets the set VG (1-3 mL/kg) and therefore the amplitude will vary as lung compliance changes. An infant on HFO is susceptible to lung volume loss or derecruitment during circuit disconnects, suctioning, or repositioning of the baby. The HFOV devices used are the Babylog VN500 and the Sensormedics 3100A  |
| HFJV | |
|---|---|
| High Frequency Jet Ventilation |
Delivers short (Ti 0.02 sec) pulses of gas to the lungs at high rates (240-600 cycles/minute or 4-10 Hz). The PEEP level is used to keep the lungs inflated and is set on the “slave” ventilator attached to the HFJV. Exhalation is passive and dependent on lung recoil. CO2 clearance is achieved by increasing PIP thereby increasing delta P (PIP-PEEP). Because the breaths are so short, the actual peak alveolar pressure is < 10% of that set. Routine patient procedures like suctioning (only performed by an RT) and patient repositioning often require Lung Recruitment Maneuvers and Jet setting alterations.
|
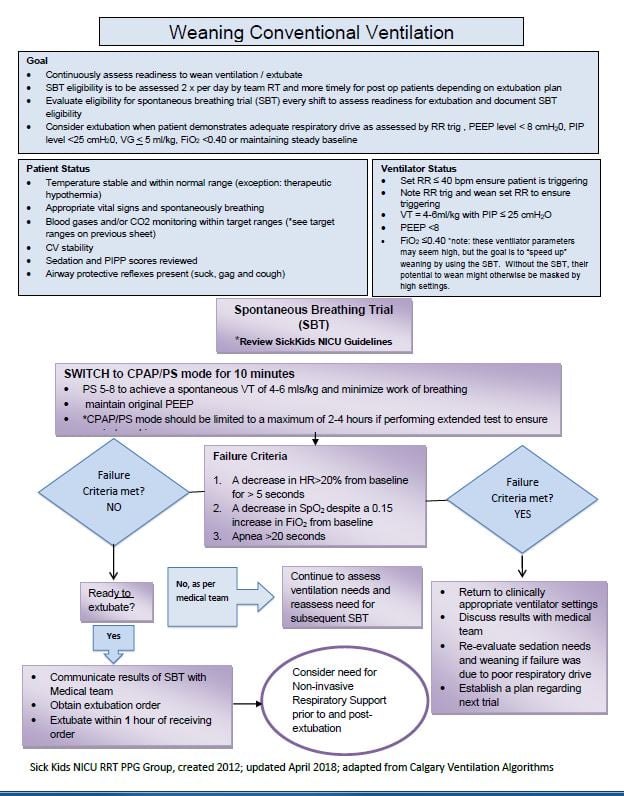
Extubation
Because of the risks of prolonged intubation and ventilation, early weaning from the ventilator to less invasive forms of ventilation is now a top priority for practising clinicians.
The use of a spontaneous breathing trial can be considered.
Post extubation therapies
CPAP
Nasal CPAP is a useful adjunct to extubation in infants when used with pressure ≥5 cm H2O. The NNT for prevention of extubation failure is 6 (95% CI 4-11).15
NIPPV
NIPPV appears to be even more successful at preventing extubation failure (NNT 3; 95% CI 2-5) than nasal CPAP.22
Methylxanthines
Best given prior to extubation for infants < 32 cGA and are effective at preventing extubation failure (NNT 4; 95% CI 2-7).23
Specific Respiratory Disorders
Table 26.13
Differential Diagnosis of Respiratory Distress in Newborn Period
| Pulmonary Disorders | ||
|---|---|---|
| Common | Less Common | |
| RDS TTN Meconium aspiration Pneumonia Pneumothorax |
Pulmonary hypoplasia Upper airway obstruction (e.g., choanal atresia) Rib cage anomalies Space-occupying lesions (e.g., diaphragmatic hernia) Pulmonary hemorrhage Immature lung syndrome |
|
| Extrapulmonary Disorders | ||
|---|---|---|
| Common | Less Common | |
| Vascular PPHN CHD Hypovolemia–anemia Polycythemia |
Metabolic Acidosis Hypoglycemia Hypothermia |
Neuromuscular Cerebral hypertension Cerebral hemorrhage Muscle or NMJ disorders Spinal cord problems Phrenic nerve palsy Drugs: morphine, phenobarbitol |
NMJ, neuromuscular junction.
We attempt to classify respiratory disorders into common and less common disorders. A combination of history, clinical examination and chest radiograph may point to one particular diagnosis.
All neonates with respiratory difficulties should have sepsis ruled out, especially, in the first hours of life as organisms such as the group B streptococcus may prove rapidly fatal; and moreover looks clinically and radiologically indistinguishable from “RDS”. Current practice is to start all infants admitted to the neonatal intensive care unit on intravenous antibiotics until sepsis can be ruled out by negative blood cultures. This however is being challenged given the evidence surrounding upsetting the human biome.
Surfactant-deficiency respiratory distress syndrome (RDS)
The commonest disorder in newborns requiring respiratory support.
Aetiology: The major problem is the inadequate maturation of the Type 2 penumocyte and the inadequate production of surfactant, which results in atelctasis. In addition the highly compliant chest wall of the preterm, increases atelectasis.
Prevention: Either delay of delivery until after 34 weeks, or the use of antenatal steroids where this is not possible. Use of betamethasone in antenatal prevention is preferable to use of dexamethasone, decreasing the incidence of PVL. A minimum of 24 hours delay in delivery with the maternal receipt of two doses is most effective. It is known that in cases of repeated dosing during episodes of threatened labour may increase risk of cerebral palsy, so more than 2 courses are not advocated.
Treatment: This has been covered under surfactant above.
Transient Tachypnoea of the Newborn (TTN)
Incidence: approximately 5 per 1000 infants delivered between 37 and 42 weeks gestation.
Aetiology: Due to delay in the clearance of fetal lung fluid. More common following delivery by caesarean section without labour.
Presentation: classically tachypnoea. Chest may appear barrel shaped. Chest X-ray shows mild hyperinflation, prominent vascular markings and fluid in the horizontal fissure.
Treatment: consider IV antibiotics to exclude infection. Most infants only need oxygen to maintain saturations; occasionally positive pressure respiratory support is required. The condition is self-limiting provided the infant is not allowed to become hypoxic which will increase pulmonary vascular resistance and may precipitate PPHN.
Meconium Aspiration Syndrome (MAS)
Incidence: Approximately 10% of pregnancies have passage of meconium at delivery; and around 5% of the infants with respiratory distress admitted to an intensive care nursery have been born through meconium stained amniotic fluid.28
Pathophysiology: Occasionally, meconium is thick leading to airway obstruction, atelectasis and “ball-valve” gas trapping. Meconium also leads to lung inflammation with protein-rich alveolar edema, with a secondary surfactant deficiency. Pulmonary hypertension often ensues.
Prevention: Definitive trials show that prophylactic intubation for meconium at delivery is inadvisable.
Therapy: The clinical problems posed are usually air-leak syndromes, or regional lung atelectasis or pulmonary hypertension – all manifested by hypoxemia. Rather than aggressive hyperventilation to reduce pulmonary hypertension, “gentle ventilation” with lower PaO2 and higher PaCO2 are maintained, has become the norm. No trials have compared different regimens of conventional mechanical ventilation in infants with MAS. Theoretically, high frequency oscillatory ventilation or high frequency jet ventilation, with its low VT and reduced pressures delivery is an attractive alternative to conventional mechanical ventilation; there have been no prospective, randomized trials comparing their respective efficacies.
The use of exogenous, natural surfactant use in these infants may be warranted.29 Inhaled nitric oxide assists in intractable pulmonary hypertension or hypoxic respiratory failure. Extracorporeal membrane oxygenation (ECMO) is rarely required for these infants And is reserved for those who do not respond to conventional therapies.
Air Leak Syndrome
Most instances of air leak syndrome, particularly those that require intervention, are complications of MV.
Anatomic locations of free gas:
- Pulmonary interstitial emphysema (PIE).
- Pneumothorax.
- Pneumomediastinum.
- Pneumopericardium.
- Pneumoperitoneum.
- Disseminated intravascular air
Pulmonary interstitial emphysema (PIE)
PIE represents free air in the interstitial spaces of the lung. Radiographically, PIE is recognized by numerous small “bubbly” air collections. Treatment is aimed and reducing peak inspiratory ventilator pressure and/or tidal volume or switching from conventional MV to HFOV. Blood gas goals should be re-targeted towards permissive hypercarbia.
Pneumothorax
- Spontaneous pneumothorax occurs in about 1% of normal babies without apparent lung disease immediately after delivery. Pneumothorax may also occur with lung diseases of all types, including pulmonary hypoplasia, RDS, PPHN, and MAS.
- Tension pneumothorax (implies mediastinal shift and blood pressure compromise, emergency treatment including chest drain insertion)
Pneumopericardium
Rare, but important because of the acute catastrophic effects of cardiac tamponade including severe hypotension. The condition requires emergency drainage.
A Chest x-ray if able to obtain due to the rapidity of patient decompensation, mays show a characteristic with a “double line” surrounding the heart. The lines represent the two layers of the pericardium.
Pneumoperitoneum
Rare to need intervention if related to lung disease, often trans-diaphragmatic passage of air from a pneumothorax.
Pneumomediastinum
Usually a radiological finding (‘sail sign’ with the thymus raised; or anterior collection seen in lateral film), very rare to need drainage and drainage should be approached with extreme caution
Diffuse intravascular pneumatosis
If gas seen on plain films in vessels &/or heart – prognosis poor. Other that drain associated pneumothoraces, little to be done.
General management of airleaks
Preferred ventilation modes: HFJV given it uses a low MAP and low VT strategy at fast rates to achieve ventilation and oxygenation and the expiratory phase is passive unlike HFOV.
Low lung volume strategy with conventional MV, The intent is to lower the PIP and tidal volume, and to lower the mean airway pressure so that resorption of interstitial air is allowed, while adequate gas exchange is monitored. If necessary, low arterial pO2 levels (e.g., 45-55 torr), more permissive hypercarbia (e.g., PaCO2 55-65 torr) and higher FiO2 are acceptable until the CXR shows signs of airleak resolution.
Early intervention with HFJV in the course of the airleak may speed resolution given it often requires less MAP than with HFOV that has active exhalation
Pulmonary Hypertension (PPHN)
Definition: PPHN is defined as severe hypoxemia due to pulmonary artery hypertension with right-to-left shunting through a PDA and/or patent foramen ovale. It is often acute and treatable, on rare occasions it is persistent and difficult to both treat and manage for example in the event of alveolar capillary dysplasia (ACD).
During fetal life pulmonary blood flow is low due to high pulmonary vascular resistance. Normally pulmonary vascular resistance falls following birth, due to lung inflation and oxygenation. When pulmonary vascular resistance fails to fall, right to left shunting occurs through the PDA and foramen ovale. Acidosis and hypoxaemia are potent pulmonary vasoconstrictors.
- Primary PPHN is rare and presents like cyanotic congenital heart disease with little or no evidence of lung disease.
- Secondary PPHN involves one or more of the four pathogenetic mechanisms listed:
Pathophysiology and Associations of PPHN:
- Pulmonary vasoconstriction: initiated by vasoactive mediators due to hypoxaemia, acidosis, sepsis. Associated with any transitional lung disease (RDS, TTN, MAS, pneumonia)
- Abnormal pulmonary vascular development: hypertrophy of vascular media with extension of muscle into smaller, normally non-muscularised pulmonary arterioles e.g. with chronic fetal hypoxia.
- Pulmonary hypoplasia: often associated with hypoplastic pulmonary vasculature e.g. oligohydramnios sequence, congenital diaphragmatic hernia.
- Pulmonary vascular occlusion: associated with thrombocytopenia and nonbacterial endocardial thrombosis.
Diagnosis:
Usually thought of in near term (>32 weeks GA) but does occur in preterms also.
The starting point is severe hypoxemia (PaO2 <60 torr) – often with ‘normal’, compliant lungs
- Chest X-ray
- Pre and post-ductal SpO2
Monitors right to left shunting at ductus level. A difference of ≥10% (or >15 torr) suggests marked pulmonary hypertension. - Echocardiography
To document the presence of pulmonary hypertension, “fetal shunts” and a structurally normal heart or elevated and sometime supra systemic RV. With careful functional echo surveillance there are many other changes that can be garnered and assist in the diagnosis and treatment. - Elevated Oxygenation index (OI), >15
Useful measure to assess the intensity of respiratory support required to maintain oxygenation, particularly as a guide on when to consider adjunctive therapies such as iNO and ECMO
Treatment:
The aim of treatment is adequate oxygenation avoiding tissue hypoxemia and elevated lactates and pulmonary vasodilator therapy.
Reflecting the paucity of RCTs, there are a number of suggested approaches.
- Oxygen therapy and ventilation – maintain SpO2 93-97 to limit further shunt and keep PaCO2 low normal to avoid VILI and cerebral hypoperfusion.
- Sedate and rarely paralyze if required but the need for continued paralysis should be assessed, particularly in severe hypoxaemia.
- Antibiotics – to ensure no concomitant sepsis
- Inotropes – used to limit potential of right to left shunt (higher systemic pressure will limit PDA flow right to left) – but many will also raise pulmonary arteriolar pressures. Consider milrinone.
- To the same end aggressive IV volume priming is often used.
- Inhaled nitric oxide (iNO) – see table for dosages. While infants with preterm RDS do have elevated Pap, the use of NO is considered experimental in these infants as of yet.
- Extracorporeal Membrane Oxygenation (ECMO) – the use of ECMO may be considered after failure of above measures, including NO and HFOV or HFJV.
Considerations for ECMO may include
- > 2.5 kg, mechanical ventilation <14 days
- No significant coagulopathy or uncontrolled bleeding.
- No major intracranial hemorrhage.
- Reversible lung injury.
- Oxygenation index (OI) indications vary.
Pulmonary Haemorrhage
Aetiology: haemorrhagic pulmonary oedema.
Presentation: acute clinical deterioration and copious bloody secretions from the endotracheal tube or in the pharynx. Infant may be pale, hypotonic, shocked and have other signs of hypovolaemia. Tachycardia is usually present and a patent ductus arteriosus is normally evident. Disseminated intravascular coagulopathy and intracranial haemorrhage are common sequelae. Chest X-ray in an infant with a large pulmonary haemorrhage will show “whiteout”.
Treatment: aim to achieve cardiorespiratory stability and correct any DIC. Ventilatory management includes suction of the ETT to avoid blockage; increasing PiP to allow adequate ventilation & oxygenation; increasing the PEEP (up to 10 cm H20) and surfactant administration (to treat secondary surfactant deficiency). Hypovolaemia should be corrected (preferably with packed red cells) and hypotension judiciously corrected with the use of inotropes. Massive pulmonary oedema may warrant the use of diuretics. Sepsis may precipitate pulmonary haemorrhage therefore, following blood cultures IV antibiotics should be commenced. The patent ductus should be treated; this is often delayed until stability has been achieved because of the side effects of indomethacin.
Prognosis: In the past massive pulmonary haemorrhage was considered lethal. With improvement in intensive care the prognosis for survival has improved.
References
- Kirpalani, H., Moore, A.M. and Perlman, M., 2007. Residents handbook of neonatology. PMPH-USA
- Askie LM, Henderson-Smart DJ, Irwig L, Simpson JM. Oxygen-Saturation Targets and Outcomes in Extremely Preterm Infants. N Engl J Med 2003;349:959-67.
- Fox WW,.Dura S. Persistent pulmonary hypertension of the neonate: Diagnosis and clinical management. J Pediatr 1983;103:505-14.
- Shannon DC, Lusser M, Goldblatt A, Bunnell JB. The cyanotic infant – heart disease or lung disease. N Engl J Med 1972;287:951-3.
- Woodgate PG,.Davies MW. Permissive hypercapnia for the prevention of morbidity and mortality in mechanically ventilated newborn infants. Cochrane Database.Syst.Rev 2001;CD002061.
- Halliday HL. Natural vs synthetic surfactants in neonatal respiratory distress syndrome. Drugs 1996;51:226-37.
- Soll RF,.Blanco F. Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome. Cochrane Database.Syst.Rev 2001;CD000144.
- Broadbent R, Fok TF, Dolovich M, Watts J, Coates G, Bowen B et al. Chest position and pulmonary deposition of surfactant in surfactant depleted rabbits. Arch Dis Child Fetal Neonatal Ed 1995;72:F84-F89.
- Segerer H, Scheid A, Wagner MH, Lekka M, Obladen M. Rapid tracheal infusion of surfactant versus bolus instillation in rabbits: effects on oxygenation, blood pressure and surfactant distribution. Biol Neonate 1996;69:119-27.
- Gregory GA, Kitterman JA, Phibbs RH, Tooley WH, Hamilton WK. Treatment of the idiopathic respiratory-distress syndrome with continuous positive airway pressure. N.Engl J Med 1971;284:1333-40.
- Elgellab A, Riou Y, Abbazine A, Truffert P, Matran R, Lequien P et al. Effects of nasal continuous positive airway pressure (NCPAP) on breathing pattern in spontaneously breathing premature newborn infants. Intensive Care Med 2001;27:1782-7.
- Miller MJ, Difiore JM, Strohl KP, Martin RJ. Effects of nasal CPAP on supraglottic and total pulmonary resistance in preterm infants. J Appl Physiol 1990;68:141-6.
- Cotton RB, Lindstrom DP, Kanarek KS, Sundell H, Stahlman MT. Effect of positive-end-expiratory-pressure on right ventricular output in lambs with hyaline membrane disease. Acta Paediatr Scand 1980;69:603-6.
- Verder H, Robertson B, Greisen G, Ebbesen F, Albertsen P, Lundstrom K et al. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. Danish-Swedish Multicenter Study Group. N.Engl J Med 1994;331:1051-5.
- Zhang S, Garbutt V, McBride JT. Strain-induced growth of the immature lung. J Appl Physiol 1996;81:1471-6.
- Davis PG,.Henderson-Smart DJ. Nasal continuous positive airways pressure immediately after extubation for preventing morbidity in preterm infants. Cochrane Database.Syst.Rev 2003;CD000143.
- Lemyre B, Davis PG, De Paoli AG. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for apnea of prematurity. Cochrane Database.Syst.Rev 2002;CD002272.
- Lachmann B. Open up the lung and keep the lung open. Intensive Care Med 1992;18:319-21.
- Bonta BW, Uauy R, Warshaw JB, Motoyama EK. Determination of optimal continuous positive airway pressure for the treatment of IRDS by measurement of esophageal pressure. J Pediatr 1977;91:449-54.
- Tanswell AK, Clubb RA, Smith BT, Boston RW. Individualised continuous distending pressure applied within 6 hours of delivery in infants with respiratory distress syndrome. Arch Dis Child 1980;55:33-9.
- Ogata ES, Gregory GA, Kitterman JA, Phibbs RH, Tooley WH. Pneumothorax in the respiratory distress syndrome: incidence and effect on vital signs, blood gases, and pH. Pediatrics 1976;58:177-83.
- Wong W, Fok TF, Ng PC, Chui KM, To KF. Vascular air embolism: a rare complication of nasal CPAP. J Paediatr Child Health 1997;33:444-5.
- Davis PG, Lemyre B, De Paoli AG. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database.Syst.Rev 2003.
- Henderson-Smart DJ,.Davis PG. Prophylactic methylxanthines for extubation in preterm infants. Cochrane Database.Syst.Rev 2003;CD000139.
- Flenady VJ,.Gray PH. Chest physiotherapy for preventing morbidity in babies being extubated from mechanical ventilation. Cochrane Database.Syst.Rev 2000;CD000283.
- Davis PG,.Henderson-Smart DJ. Intravenous dexamethasone for extubation of newborn infants. Cochrane Database.Syst.Rev 2001;CD000308.
- Halliday HL, Ehrenkranz RA, Doyle LW. Early postnatal (<96 hours) corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database.Syst.Rev 2003;CD001146.
- Wiswell TE. Advances in the treatment of meconium aspiration syndrome. Acta Paediatr Suppl 2001;436:28-30.
- Findlay RD, Taeusch HW, Walther FJ. Surfactant replacement therapy for meconium aspiration therapy. Pediatrics 1996;97:48-52.
- Todd DA, Wright A, Broom M, et al. Methods of weaning preterm babies <30 weeks gestation off CPAP: a multicentre randomised controlled trial. Archs Dis Childh Fetal Neonatal Ed. 2012;97:F236–40.
Appendix:
Some Pulmonary Physiology
- Compliance (unit of volume divided by unit of pressure required to move that volume), reflects how easily the lung and chest wall inflates with pressure. The full term neonate has a compliance of 3.5 to 6.0 mL/cm H2O. In respiratory distress syndrome (RDS), a lower compliance and requires a higher pressure to deliver the same volume in comparison with normal lungs.
- Dynamic compliance – measured during delivery of flow to the patient; it includes not only the compliance but also airway resistance.
- Static compliance – measured in “no-flow” situations, i.e. during a breath hold. Static compliance reflects the pulmonary and chest wall compliance, without being affected by airway resistance.
- Pulmonary Resistance: resistance to gas flow in the airways resulting in friction (pressure per unit flow rate).
- A driving pressure is needed to overcome the resistance. The nasal passages of a neonate contribute up to 40% of the total resistance of the pulmonary system.
- Time constants are the product of compliance and resistance.
- They indicate how fast or slowly the lungs will inflate or deflate. A neonate with RDS has a very “fast” expiratory time constant, meaning the patient exhales to equilibrium very quickly.
- One time constant is needed to equilibrate 63% of the gas and five times the time constant for 95% of the gas.
- A corollary is that very fast respiratory rates can be used with neonates with RDS, whereas babies with meconium aspiration have higher airway resistance. The high airway resistance gives the baby a slow time constant and short expiratory times can lead to gas trapping.
- The respiratory equation of motion indicates that all these variables are highly interdependent – changing one variable will perforce alter the others:
Prespsytem = 1/ CRS x volume + RRS x flowThis states that the amount of pressure generated (respiratory system here means patient and ventilator) to deliver a tidal volume:
is equal to the pressure necessary to move a volume against the reciprocal of compliance of respiratory system.
plus the pressure necessary to move the associated flow through the respiratory resistance (here circuit and lung resistances)