Nephrologic Disorders
Aoife M. Waters MB, MRCPI and Tino D. Piscione MD, PhD, FRCP(C), Damien G. Noone MB BCh BAO, MSc.
The material presented here was first published in the Residents’ Handbook of Neonatology, 3rd edition, and is reproduced here with permission from PMPH USA, Ltd. of New Haven, Connecticut and Cary, North Carolina.
Evaluation of Neonatal Renal Function
A. Chronology Of Structural Development
| 5 weeks: | structural kidney development begins |
| 9 weeks: | initial urine formation |
| 16-25 weeks: | most rapid rate of nephrogenesis |
| 32-34 weeks: | nephron formation completed |
In newborns < 34 weeks gestation, the rate of nephrogenesis ex utero is not accelerated post-natally and in extremely preterm infant’s nephrogenesis stops about 40 days after birth.
Note: normal kidney development is a highly dynamic process of cell proliferation, cell death, morphogenesis and differentiation controlled by many genes. Renal malformations, such as agenesis (failure of formation of the metanephros, week 5) and dysplasia (failure of normal renal differentiation) may result in fetal oliguria, oligohydramnios and pulmonary hypoplasia (discussed in Section III).
B. Urine Volume
Dynamics in Utero
- The 2 primary sources of amniotic fluid production are fetal urine (75%) and lung liquid (25%).
- The 2 primary routes of amniotic fluid removal are fetal swallowing (66%) and intra-membranous (i.e. “trans-fetal”) re-absorption (33%).
Note: the mean amniotic fluid index (AFI) in pregnancy is about 12-14 cm and after 33 weeks it declines to a mean of 12 cm at term. Polyhydramnios implies an AFI > 25 cm, moderate oligohydramnios an AFI of 5 – 8 cm and severe oligohydramnios an AFI < 5 cm).
C. Creatinine clearance
Serum creatinine at birth is reflected by maternal creatinine.
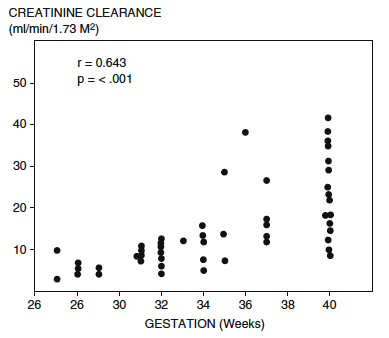
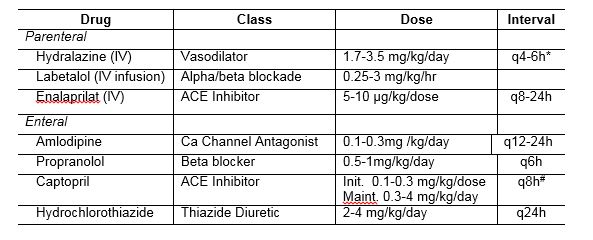
Figure showing creatinine clearance measured within 24-40 hours of birth highlighting the diminished clearance of extreme prematurity. Chevalier RL. Developmental renal physiology of the low birth weight preterm newborn. J Urol. 1996
Serum creatinine at birth reflects the initial low creatinine clearance. To convert the creatinine from mg/dL to mol/L multiply by the conversion factor 88.4. Stonestreet BS, Oh W. Plasma creatinine levels in low-birth-weight infants during the first three months of life. Pediatrics. 1978
As compared to term neonates, the delayed drop in serum creatinine following the first few days of life in premature infants reflects:
- Fewer number of functioning glomeruli.
- Reduced surface area & permeability of glomerular filtration barrier.
- Diminished intra-glomerular Starling Forces (i.e.. lower intra-glomerular hydrostatic pressure, lower plasma oncotic pressure).
D. Glomerular Filtration Rate (GFR)
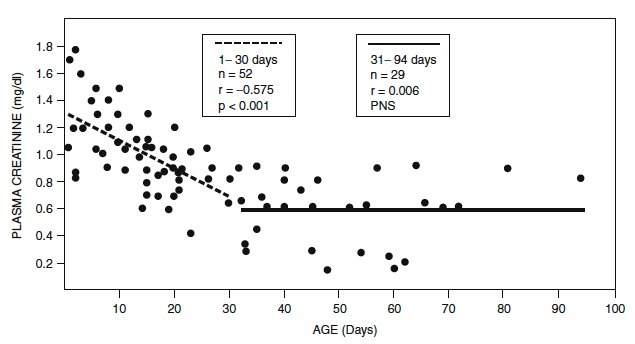
- During fetal life, GFR increases progressively from 14 ml/min/1.73 m2 at 32–34 weeks’ gestation to 21 ml/min/1.73 m2 at term.
- GFR doubles by 2 weeks of age.
- Adult values of 118 ml/min/1.73 m 2 are achieved by the age of 2 years.
- Rate of GFR maturation is a function related to postconceptional age and not postnatal age, and the postnatal rise in GFR in preterm infants is slower (Figure)
Nephrology and fluid/electrolyte physiology: neonatology questions and controversies. [edited by] William Oh MD and Michel Baum MD
Philadelphia, PA : Elsevier Inc, c2019. Third edition. (Modified from Guignard JP, John EG. Renal function in the tiny, premature infant. Clin Perinatol. 1986;13:377.)
E. “Normal” Urine Output in Perinatal Period (Table 3)
→ 3 Phases Of Physiologic Perinatal Diuresis: reflects changes in GFR, renal blood flow, total body water compartment shifts
1. Prediuretic phase:
- Variable, usually 12 to 24 hours of life.
- Reflects low GFR, high renal vascular resistance.
- Full term (approx.1 cc/kg/hr); premature infant (0.5 to 1 cc/kg/hr).
2. Diuretic phase:
- Between 1 to 4 days of life (duration and magnitude of diuresis is inversely proportional to gestational age at birth).
Table 18.1.
Mean plasma creatinine values in the perinatal period for different gestational ages (derived from (1)).
Plasma creatinine values are quoted in SI units.
To convert to the conventional units (mg/dl), divide by the conversion factor 88.4
| GESTATIONAL AGE (weeks) | POST-NATAL AGE | |||
| 2 days
(µmol/L) |
7 days
(µmol/L) |
14 days
(µmol/L) |
28 days (µmol/L) |
|
| 28 | 116 | 84 | 72 | 58 |
| 29-32 | 104 | 83 | 69 | 52 |
| 33-36 | 93 | 68 | 55 | 35 |
| 37-42 | 75 | 50 | 38 | 30 |
Table 18.2
Determinants of normal neonatal Glomerular Filtration Rate (GFR)
| IN-UTERO | POST-NATAL | |
| Renal Blood Flow | LOW
(2.5-5% cardiac output) |
Doubles by 2 weeks in newborns,
reaching mature levels by 2 years of age.
|
| Renal Vascular Resistance | HIGH
· High circulating catecholamines · High renin angiotensin system activity |
LOW |
| CONSEQUENTLY GFR | LOW
(1ml/min/kg in the last trimester) |
INCREASES – doubles by 2 weeks of age |
GFR = glomerular filtration rate. Note renal blood flow in adult
Kidney is ~ 1 L/min (20% of cardiac output)
Table 3. Maximum urine concentration capacity in the newborn
| Gestational Age | Maximum Urine Osmolality |
| 24 weeks | 100-200 mOsm/kg |
| 40 weeks | 600-800 mOsm/kg |
| Adult | 1200 mOsm/kg |
The capacity to concentrate urine increases progressively during postnatal life. A diminished responsiveness of the collecting ducts to vasopressin (ADH) due to low expression of aquaporin water channels, responsible for water reabsorption in the collecting duct, is one of the most likely mechanisms for this reduced concentrating capacity of the neonatal nephron.2 The expression increases postnatally and is associated with a progressive increase in urinary concentrating capacity.
- Reflects rise in GFR, drop in renal vascular resistance, raised extracellular volume (ECV).
- Full term (1.5-fold increase) in urine output
- Premature infant (up to 3-fold increase) in urine output.
- Acceptable body weight loss: 1 to 2% body weight/ d eg, in a < 1,000 g infant, £ 20g/day
- Salt loss (high FeNa ® reflects expanded ECV).
- B. diuresis and natriuresis ® reflects ISOTON-
IC fluid loss; therefore, water and salt excretion during this period does not accompany a change in plasma circulatory volume or serum [Na].
3. Postdiuretic phase:
- Reflects maturational capacities for sodium and water reabsorption.
- Urine output and FeNa returns to prediuretic levels.
- Premature infants are susceptible to a negative water and salt balance because they have a low capacity to retain sodium and their renal excretion of sodium is usually high.
Note: Urine diluting capacity in neonates is the same as adults – 50 mOsm/L, however, their ability to excrete a water load is initially limited due to the lower GFR, so they can be prone to water overload and dilutional hyponatremia.
F. Sodium Excretion
A. Fractional excretion of Sodium (FENa):
FENa (%) = (UNa / PNa) x (PCreat / UCreat) x 100
FENa @ 32 weeks 3-5%
FENa @ term <1%
UNa = urinary sodium (mmol/l)
PNa = plasma sodium (mmol/l)
PCreat = plasma creatinine (umol/l)
UCreat = urinary creatinine (mmol/l)
Note: Preterm infants require more sodium supplementation (3-5 mmol/kg/day) and preterm breast milk has higher sodium than term breast milk (25 mmol/L versus 7 mmol/L)
B. Sodium Balance In The First Days Of Life:
Day 1-4:
- Physiologic natiuresis → commensurate with ECV expansion
- Isotonic Na loss
- Sodium supplementation not required during this phase.
Day 5+:
- Premature infants may exhibit diminished capacity for tubular Na reabsorption (severity proportional to prematurity).
- Estimated Na OUT = urinary loss
e.g. Daily Na+ requirement = UNa+ (mEq/L) x Urine flow rate (cc/kg/hr) x 24hr
≈ 4 mEq Na+/kg/d
Diagnostic approach and management of acute kidney injury (AKI) in the newborn
- Incidence of AKI approx. 8% – 24% of severely ill newborns. Rates are much higher in asphyxiated newborns undergoing cooling/therapeutic hypothermia (38%), infants with congenital heart disease requiring surgery (52%), or infants with congenital diaphragmatic hernia needing ECMO (71%). ( Bryan Carmody and Jennifer R. Charlton. Pediatrics 2013;131;1168)
- Neonatal AKI associated with high mortality risk : 25 to 50% of babies with AKI will 3 See Tables 4 through 7. There is a 2-3 times increased odds of death with AKI. If creatinine increases by 1 mg/dL (88 mmol/L) the adjusted OR 3.44, 95% CI 1.23 – 9.61.
- The major risk factors for AKI in ELBW infants in the largest study to date include mean airway pressure > 10 cm H2O (Adj OR 4.8), mean arterial pressure < 21 mmHg (Adj OR 8.1) and use of cefotaxime (Adj OR 5.26). (Viswanathan et al. Ped Nephr (2012) 27;303-311)
A. Definition Of Oliguria: Urine output < 0.5cc/kg/hr
N.B. 7% of normal neonates do not empty their bladders in the first 24 hours of life.
B. Definition Of AKI:
(N.B. 30% of newborns with renal impairment may present with nonoliguric AKI eg, obstructive uropathy, severe asphyxia).
- Rising serum creatinine (see table 2 for gestation-associated serum creatinine ranges) ± oliguria.
- Stage 1 – ↑ SCr by 0.3 mg/dl (27μmol/L) or ↑ to ≥ 150-200% from baseline
- Stage 2 – ↑ to ≥ 200–300% from baseline
- Stage 3 – ↑ to ≥ 300% from baseline
C. Classification of AKI based on Urine Output:
- Oliguric renal failure is defined as having a urine output < 1 ml/ck/hour that is unresponsive to a fluid challenge and the serum creatinine is > 1.5 mg/dL or 133 mol/L.
- Non-oliguric AKI implies a urine output of > 1 ml/kg/hour is maintained but the creatinine is elevated > 1.5 mg/dL or 133 mol/L.
D. Management of Acute Renal Failure:
- Accurate monitoring is the key to manage- ment. Weigh q8–12 h. Note presence of edema, increased abdominal girth, peripheral edema, etc.
- Restrict fluid input to insensible fluid loss plus ongoing losses.
Table 4.
Causes of acute renal failure in the neonatal period.
| Prerenal (85%) | Intrinsic Renal (11%) | Post-renal (4%) |
| Decreased ‘True’ Intravascular Volume
Total Body Water Depletion |
Congenital | Congenital |
| Dehydration, Haemorrhage, GI losses (NEC), Third space losses (sepsis) | Dysplasia, Aplasia, Polycystic kidney disease, Rare syndromes | Posterior urethral valves, Uretero-pelvic junction obstruction, Prune belly syndrome, Tumours |
| Decreased ‘Effective’ Intravascular Volume, Total Body Water Expansion | Acquired | Acquired |
| Congestive cardiac failure, Respiratory failure, Hepatorenal syndrome, Asphyxia, Indomethacin | Maternal drugs (eg ACE inhibitors), COX inhibitors, Acute tubular necrosis, Renal vein thrombosis, Pyelonephritis, Other nephrotoxic drugs | Fungal Ball, Neurogenic Bladder, Spinal dysraphism, Asphyxia (neurogenic bladder retention) |
ACE = angiotensin-converting enzyme; GI = gastrointestinal; NEC = .
- Insensible losses: Full term: 30 mL/kg/day. Preterm: 50 to 70 mL/kg/day.
- Ongoing losses: urine (mL/kg/h); plus other losses, eg, drainage effusions, diarrhea. Calcu- late all inputs, including infusions in arterial line. In the polyuric phase following postrenal ARF.
Table 5.
Clinical indices of acute renal failure in the newborn infant.
| Clinical Status | |
| Weight changes | Day 1-5: < 5% weight loss AND clinical signs of fluid overload |
| Volume status | Persistent hypertension, Tachycardia, Edema, Hypoperfusion |
| Physiologic Measurements | |
| Urine output | Anuria: ensure bladder empty, Oliguria:
FT: < 1cc/kg/h Extreme Prem.: < 0.5cc/kg/h on day 1; Day 2+, < 1 cc/kg/h |
| Urinary Na+ | Prerenal: < 20 meQ/L
Intrinsic: > 20 meQ/L |
| FENa | Prerenal < 1%; <3% in premature infants.
Intrinsic (e.g. ATN) > 10% Spot urines unreliable in infants, esp. LBW, and following diuretic use; but may be necessary to use as guide. |
| Serum K+ | FT: > 6.5 @ birth, or failure to decrease over 5 days. |
ATN = ; FT = ; LBW = .
it may be necessary to replace measured urine losses q4–6h.
- For effective fluid restriction, consider three main goals:
- Maintain euglycemia: If this is possible at intra- venous (IV) solution concentrations of <10% glucose, it is safe to use peripheral lines. To avoid hypoglycemia, may need IV solutions with glucose concentration of 20% with central ve- nous access. With D20W running at 60 mL/kg/ day, glucose input is about 8 mg/kg/min.
Table 6. Radiographic investigations in neonatal acute renal failure.
| Investigation | What are you looking for? | Timing |
| Ultrasound | Parenchyma
Urinary tract
|
When renal failure suspected. |
| Doppler | Vascular
|
Note: preterms and neonates in the first few days of life have a physiologically higher resistive index and relatively lower diastolic flow velocity. |
| Radionuclide scan | Dynamic assessment of
|
Depends on course of renal impairment
|
| 99m Tc-MAG-3 | Tubular excretion function
Recommended over DTPA for neonates → more precise correlation with function,
|
|
| 99m Tc-DTPA | GFR
Not recommended < 3 months of age. |
|
| 99m Tc-DMSA | Static assessment:
|
6 months post-recovery to determine residual scar
(DMSA not recommended before 12 weeks post-natal age) |
| VCUG | VU Reflux
Unilateral vs. bilateral Bladder size, shape Large, trabeculated → lower urinary tract obstruction Small, smooth → upper tract obstruction |
Immediate, if antenatal findings on US include bilateral hydronephrosis, full bladder, and oligohydramnios |
* refers to anteroposterior diameter of renal pelvis
ATN = ; DMSA = ; DTPA = ; GFR = glomerular filtration rate (; RVT = ; VU =.
Table 7. Mean Renal Lengths By Ultrasonography for Various Gestational Ages 4
| Gestational Age (weeks) | Mean Fetal Renal Length (95% C.I.)(cm) |
| 28 | 2.6-4.2 |
| 32 | 3.1-5.1 |
| 34 | 3.3-5.0 |
| 36 | 3.3-5.0 |
| 40 | 3.2-5.3 |
G. Management Of Acute Renal Failure
1. Monitoring And Inputs Of Fluid And Electrolytes
A. Accurate monitoring is the key to management. Weigh q8-12 h. Note presence of edema, increased abdominal girth, peripheral edema, etc.
B. Restrict fluid input to insensible fluid loss plus ongoing losses.
-
- Insensible losses: Full term: 30 ml/kg/day. Preterm: 50-70 ml/kg/day.
- Ongoing losses: urine (ml/kg/h); plus other losses, e.g., drainage effusions, diarrhea. Calculate all inputs, including infusions in arterial line. In the polyuric phase following post-renal ARF, it may be necessary to replace measured urine losses q4-6h.
C. For effective fluid restriction, consider three main goals:
-
- Maintain euglycemia: If this is possible at IV solution concentrations of <10% glucose, it is safe to use peripheral lines. To avoid hypoglycemia, may need iv solutions with glucose concentration of ≥ 20%, with central venous access. With D20W running at 60 ml/kg/day, glucose input is about 8mg/kg/min.
- Maintain near to isonatremia: Sodium additions to high glucose solutions can be made up with 3% sodium chloride (0.5 mmol/ml) or 8.4% sodium bicarbonate (1 mmol/ml).
- Minimize potassium rises: Potassium should not be added until urinary output well established.
In infants with ARF, fluid orders should be re-evaluated q8-12 h initially, and revised on the basis of all available information.
D. Serum electrolytes: q6-12h.
E. Acid-base measures: q6-12h.
F. Serum urea, creatinine:
G. Serum Ca, Mg: daily, or more often if low.
H. Spot urine electrolytes: may be used to estimate urinary sodium losses.
I. Urinalysis daily, to monitor for blood, protein.
J. Other ongoing losses: e.g. ostomy losses → measure electrolyte concentrations and volumes.
K. Losses such as “third spacing”: cannot be measured, but may be estimated by monitoring weight changes.
2. Medical Therapy Specifically Directed At Cause
A. Prerenal Renal Failure:
-
- Support blood pressure to minimize any ongoing renal hypoperfusion
- Support oxygenation → incipient pulmonary edema may dictate more aggressive intervention (e.g. dialysis).
B. Intrinsic Renal Failure → Assess exposure to nephrotoxins:
-
- Consider the possibility that any drugs the infant is receiving may be nephrotoxic.
- Consider discontinuing potentially nephrotoxic drugs if non-essential.
C. Postrenal obstruction:
-
- If large bladder is found on palpation and/or ultrasonography, bladder catheterization should result in large volumes of urine. Consider posterior urethral valves (mainly boys) or neurogenic bladder (e.g. HIE). Rarely, enlarged bladder may be associated with a spina bifida occulta.
- Urinary tract infection (UTI). Often Gram negative rods, especially E. coli. -In these cases must exclude galactosemia. Urosepsis often associated with congenital urinary tract obstruction.
3. Nonspecific Medical Therapy
A. Minimize drug use. Examine all prescribed and other drugs for potentially nephrotoxic agents. Consult drug formulary to determine dose or duration modification required for renal impairment. In the context of acute renal failure, assume severe impairment. If nephrotoxic drugs must be used, guide dosing schedule according to therapeutic drug monitoring guidelines.
B. Further electrolyte and nutrient therapy:
-
- Potassium: withhold from intravenous fluids initially.
- Management of hyperkalemia:
- ECG monitoring.
- Correct calcium and magnesium concentrations to minimize adverse effects on heart.
- B. Sodium-potassium exchange resins (e.g. kayexalate) administered by nasogastric tube or rectally may cause bezoar formation.
- When using insulin-glucose infusion, be vigilant against hypoglycemia.
- Restrict phosphates.
- Restrict protein to £ 0.5 g/kg day as essential amino acids.
In practice both phosphate and protein inputs are often not given in the initial treatment phase especially when input is restricted to glucose and tightly controlled sodium solutions. - Acidosis: If acidosis is present, administer sodium bicarbonate. Serum Na+ monitoring is recommended as a precaution against hypernatremia.
- Theophylline, an adenosine antagonist that may reverse intrarenal vasoconstriction may prove to be beneficial in AKI in asphyxiated infants although it is still not recommended.
4. Renal Replacement Therapy
May consist of pure dialysis, pure filtration or both.
Dialysis: removal of small solutes by diffusion across a semipermeable membrane down their concentration gradient
The membrane may be peritoneum, separating blood in the splanchnic capillaries from dialysis fluid within the peritoneal cavity, or it may be synthetic as in haemodialysis, blood and dialysis fluid being pumped on opposite sides of the membrane.
Ultrafiltration is convective removal of plasma water and small solutes as a result of a pressure gradient across a highly permeable membrane.
Routes: See Table 8
Indications:
- Life-threatening hyperkalemia (arrythmias, K > 8.5mmol/L)
- Persistent, severe metabolic acidosis.
- Diuretic-resistant volume overload
- Intractable hypertension caused by volume overload
- Inability to meet nutritional needs in an oliguric/anuric patient
- Severe azotemia (BUN > 40)
- Overdose of a dialyzable toxin
Note that a rising creatinine per se is not an indication for dialysis.
Ethical considerations:
- Before embarking on dialysis, ethical factors must be considered with a multidisciplinary team comprised of pediatricians nephrologists, social workers, nurses in consultation with the parents. Consider the following:
- Is the renal defect reversible?
- How long is dialysis likely to be needed?
- What are the other medical problems (short and long term) faced by the infant?
- What is the likely prognosis ? What are the parental views?
- Several meetings, preferably multidisciplinary, will likely be needed to answer these questions adequately. Questions may arise with reference to the degree of discomfort/pain etc. that the infant may experience. It is not uncommon practice to institute peritoneal dialysis in the infant >1500g when the likelihood for survival without severe neurologic disability and complete recovery of renal function is high.
- Where these conditions are not met, decision making is more difficult; hence the need for full and careful discussions.
- Cranial imaging using various modalities and electrophysiologic testing is indicated in infant with multiple anomalies, HIE, or neurologic signs.
5. Survival on dialysis
- Data from the US suggests that in those who initiate dialysis under the age of 1, 81.9% are alive at 12 months, 73.2 % at 2 years and 65.4% at 3 years (Zaritsky J, Warady BA. Peritoneal Dialysis in Infants and Young Children. Seminars in Nephrology, Vol 31, No 2, 2011)
- Data from Australia showed that the 5 year survival of those who started renal replacement therapy under age 1 was 73%. McDonald SP, Craig JC (2004) Long-term survival of children with end-stage renal disease. N Engl J Med 350:2654–2662)
- A pan Canadian study found that the mortality is greatest if renal replacement therapy is started under 3 months of age at 36.7%.
Table 8.
Features of neonatal renal replacement therapy modalities.
| Peritoneal Dialysis | Hemodialysis | Continuous Veno-venous Renal Replacement Therapy (e.g. CVVH) | |
| Access | Acute, temporary:
– Non-tunnelled – 5 Fr Cook catheter Permanent: – Tunnelled – Infant swan neck coil cuff catheter |
Acute, temporary:
– Non-tunnelled – Femoral or IJ* – 6.5Fr Gamcath or 2 Cardiomed 16g Permanent: – Tunnelled – IJ – 8Fr Medcomp Hemocath |
Acute, temporary:
– Non-Tunnelled femoral or IJ – 6.5Fr Gamcath |
| Need for systemic anti-coagulation | No.
Heparin may be added to dialysate bags (500 U/L) if fibrin fragments evident in effluent. |
Yes, during therapy.
May be performed heparin-free; however, high risk of circuit clotting and loss of extra-corporeal blood when no anti-coagulation used. |
Yes.
High risk of circuit clotting and loss of extra-corporeal blood when low level anti-coagulation used. |
| Rate of Solute Removal | Slow, continuous.
Not recommended for solute clearance related to primary metabolic disease. |
Rapid.
Recommended for solute clearance related to primary metabolic disease, hyperammonemia. |
Slow, continuous.
Recommended for solute clearance related to primary metabolic disease, hyperammonemia. |
| Rate of Fluid removal | Slow, continuous.
Regulated by glucose content of dialysate. Recommended in hemodynamically unstable patients. |
Rapid.
Regulated by UF pump on dialysis equipment. Not recommended in hemodynamically unstable patients. |
Slow, continuous.
Regulated by UF pump on dialysis equipment. Recommended in hemodynamically unstable patients. |
| Complications | Cardiovascular instability.
Inadequate UF and clearance. Hyperglycaemia. Catheter malfunction. Inflow/outflow obstruction. Hydrothorax. Hypothermia. Peritonitis. Scrotal/labial oedema. |
Access failure.
Clotting of circuit. Hypothermia. Hypotension. Sepsis. Bleeding secondary to systemic anticoagulation. Air embolism (usually fatal). Tubing disconnection with bleeding. Anaphylaxis to filter membrane. Exposure to blood products (blood prime required due to high extracorporeal circuit volume). |
Frequent clotting.
Inadequate uremia control in severely catabolic patients. Bleeding secondary to systemic anticoagulation. Infection. Filter rupture. Tubing disconnection with bleeding. Hypophosphatemia, hypokalaemia. Drug removal. Air embolism. Hypothermia. Hyperglycemia. Exposure to blood products (blood prime required due to high extracorporeal circuit volume). |
| Contraindications | Omphalocele, gastroschisis
Diaphragmatic hernia Bladder exstrophy Relative: – Recent abdominal surgery – Intra-abdominal malignancy |
Hemodynamically instability.
Birth weight < 1.5 kg (catheter size limitation). Grade II-IV IVH Relative: – Grade I IVH – Uncontrolled bleeding |
Hemodynamically instability.
Birth weight < 1.5 kg (catheter size limitation). Grade II-IV IVH Relative: – Grade I IVH – Uncontrolled bleeding |
* Internal jugular vein
Diagnostic approach and management of congenital renal malformations.
Epidemiology 5, 6
-
- Overall rate of renal and urinary tract defects in live and stillborn infants: 3-1.6 per 1000.
- Renal malformations account for approximately 20-30% of all antenatally detected congenital anomalies.
- 48% of infants diagnosed with a renal malformation exhibit more than one structural renal anomaly.
- 89% of infants with renal anomalies exhibit extra-renal birth defects.
- 18% of infants with renal dysplasia have vesico-ureteric reflux.
- Over 100 syndromes are associated with renal and urinary tract malformations.
- Note that many cases of pelvic dilatation detected on static antenatal ultrasound ‘resolve’, and repeat scans are advised.
Table 8.
Morphologic classification of renal malformations.
| Anomalies of Renal Formation in toto | Bilateral renal agenesis (1:5000; ♂:♀=2:1)
Unilateral renal agenesis |
| Anomalies of Renal Position and Form | Renal hypoplasia
Renal fusion (e.g. horseshoe kidney) Renal duplication (e.g. duplex kidney) Renal ectopia (e.g. pelvic kidney) |
| Anomalies of Renal Parenchymal Differentiation | Congenital hydronephrosis
Multicystic dysplasia (often unilateral) Renal tubular dysgenesis Polycystic Kidney Disease · Autosomal Recessive · Autosomal Dominant |
| Anomalies Associated With Lower Urinary Tract Malformations | Obstructive dysplasia associated with:
· Posterior Urethral Valves · UPJ Obstruction · Neurogenic Bladder · Ureterocoeles · Prune Belly Syndrome · Primary Vesico-ureteric Reflux |
Most common (in decreasing order):
- Congenital hydronephrosis (i.e. non-dysplastic pelvicalyceal dilatation)
- UPJ Obstruction
- Renal agenesis/dysplasia
- Multicystic dysplastic kidneys
- Pelvic/horseshoe kidneys
- Posterior Urethral Valves
Poor Prognostic Findings on Antenatal Ultrasound
- Small renal size
- Bilateral hydroureteronephrosis
- Cysts
- Increased echogenicity
- Bladder dilatation
- Oligohydramnios
Oligohydramnios
-
- Causes:
- Bilateral renal agenesis
- Bilateral polycystic kidney disease, infantile type
- Complete urinary tract obstruction
- Placental insufficiency
- Features of Oligohydramnios Sequence/Potter’s Sequence
- Obstetric history: history of pregnancy loss, gestational diabetes, exposure to teratogens (e.g. vitamin A, ACE Inhibitors).
- Genetic history: family history congenital malformations (incl. renal) or syndromes, family history kidney disease (e.g. ADPKD)
- Amnion nodosum on the placenta (almost pathognomonic of oligohydramnios due to renal anomalies)
- Potter’s facies:
- Pseudoepicanthus.
- Recessed chin
- Posteriorly rotated, flattened ears.
- Flattened nose.
- Associated musculoskeletal features:
-
-
- Clubfoot and clubhand.
- Hip dislocation.
- Joint contractures
- Broad, flat hands.
-
-
- Sonographic appearance of kidneys
- Absent.
- Tiny
- Normal.
- Enlarged, palpable.
- Lungs
- Pulmonary hypoplasia
- Chest X-ray: small bell-shaped chest, lung fields well-aerated despite small lung volume.
- Pneumothorax.
- Causes:
Diagnostic and Therapeutic Approach in Suspected Congenital Renal Malformation
Fetal renal anomalies can be ascertained from:
- Positive family history of renal anomalies.
- Increasing hydronephrosis on serial antenatal sonography
- Hydronephrosis initially detected on routine antenatal sonography in the third trimester.
- Altered amniotic fluid volume.
Key Findings To Note On Post-natal Physical Exam:
- Poor urinary stream
- Presence of a flank mass
- Survey for other congenital anomalies
Medical Management
- Male sex: If strongly suspicious of severe congenital renal malformation (e.g. posterior urethral valves), attempt urinary bladder catheterization.
- Catheter size recommendations:
- Newborn: 5, 6, or 8 Fr Foley catheters (feeding tube acceptable, but they may become easily dislodged).
- Premature: 5 or 6 Fr Foley
- Strict Ins and Outs. B. Infants with obstructive uropathy may show high urine volumes. In this instance, fluid requirements may exceed “maintenance” levels to keep up with high urine output. Therefore, total fluid intake should consider insensible and urinary fluid losses.
- Obtain urine culture. Full septic work-up if clinical suspicion of sepsis is high. If clinical suspicion of sepsis is high, prescribe ampicillin and gentamicin to cover potential urosepsis.
- Adjust drug dosing according to guidelines suggested in the context of acute renal failure.
- Lab Investigations: Close monitoring of serum creatinine and electrolytes advised in the context of:
- Severe lower urinary tract obstruction (e.g. posterior urethral valves, prune belly syndrome)
- Bilateral hydronephrosis
- Solitary kidney with unilateral hydronephrosis
Timing of Radiographic Investigations When Renal Malformation Suspected:
- Early (within 24 hours of life), when the following antenatal findings are evident:
- Progressive antenatal hydronephrosis.
- Distended bladder on antenatal sonograms.
- Bilateral severe antenatal hydronephrosis.
- Any one of the above with male sex.
- Later (Day 3-7 of life), when in the context of the following antenatal findings:
- Stable, non-progressive antenatal hydronephrosis.
- Unilateral antenatal hydronephrosis.
- 2 normal-sized kidneys
- Normal amniotic fluid volume
- Female sex.
Figure 2.
Approach To Radiographic Investigations In The Neonatal Intensive Care Unit.
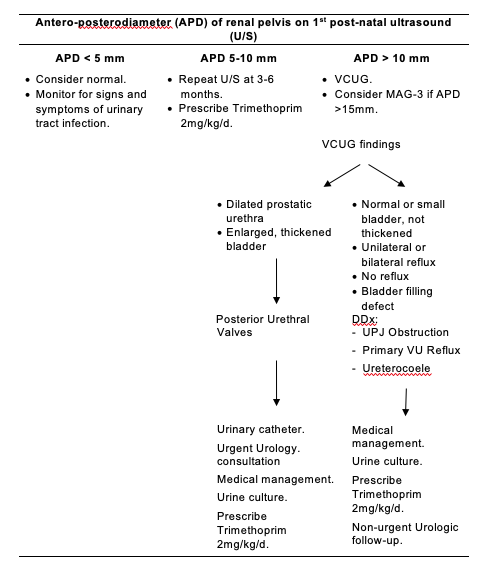
Note:
Antenatal hydronephrosis is associated with vesico-ureteric reflux, or upper or lower urinary tract obstruction in 14-21% of infants and therefore trimethoprim prophylaxis should be considered against urinary tract infection until further investigations such as VCUG and follow up renal ultrasound to assess for progression of hydronephrosis are performed. If there is no reflux and resolution of the hydronephrosis or nonprogression on follow up renal ultrasound scans without interim urinary tract infection, then consider stopping prophylactic antibiotic cover. However, if there is reflux or progression of hydronephrosis, then one should continuing prophylaxis.
Diagnostic approach and management of microhematuria in the newborn
Definition of hematuria
5 RBCs per high power field on more than 3 occasions.
NB Distinguish between urates: no RBCS on microscopy
Table 9.
Common causes of microhematuria in the newborn and pertinent historical and physical exam findings.
| Cause Of Microhematuria | Pertinent Point On History And Physical Exam | Investigations and Management |
| Urethral trauma | Urinary catheter | Remove catheter if possible. |
| Urinary tract infection | Urinary catheter
Sepsis Known congenital urogenital malformation. |
Urine culture.
Treat urinary tract infection. Ultrasound and VCUG. |
| Acute tubular necrosis, cortical necrosis | Perinatal asphyxia
Perinatal hemorrhage |
Manage as per level of renal impairment.
Monitor for proteinuria. |
| Hypercalciuria, nephrocalcinosis | Medications
|
Discontinue medications. |
| Urogenital malformations | History of antenatal hydronephrosis or renal anomalies.
Palpable flank mass. Palpable bladder. Hypertension. |
Ultrasound.
VCUG. Manage as per level of renal impairment. |
| Renal mass | Palpable flank mass.
Hypertension. |
Ultrasound.
Surgical consultation. Oncology consultation. |
| Renal venous thrombosis | Maternal diabetes.
Perinatal hemorrhage. Umbilical vessel catheterization. Dehydration. Patent ductus arteriosus. Thrombocytopenia. Polycythemia. |
Remove umbilical lines.
If bilateral, or if abnormal renal function, consider anticoagulation with systemic or local thrombolytics. Note: Silent disease may remain undiagnosed and small thromboses may undergo spontaneous remission. If unilateral and if normal renal function, follow with serial US. |
| Coagulopathy | Sepsis.
Hemorrhage. |
CBC, LDH, haptoglobin.
INR/PTT, D-dimers, Fibrinogen Treat underlying cause. FFP, platelet transfusion as necessary. Maintain good diuresis to prevent heme cast nephropathy. |
| Myoglobinuria | Severe asphyxia.
Status epilepticus. |
Urine myoglobin.
Serum CPK measurement. Maintain good diuresis (>2cc/kg/hr) |
CBC = ; CPK = ; D-dimers = ; INR/PTT = ; LDH = ; US = ; VCUG = .
Figure 3. Diagnostic Algorithm for Microscopic Hematuria in the Newborn Infant.
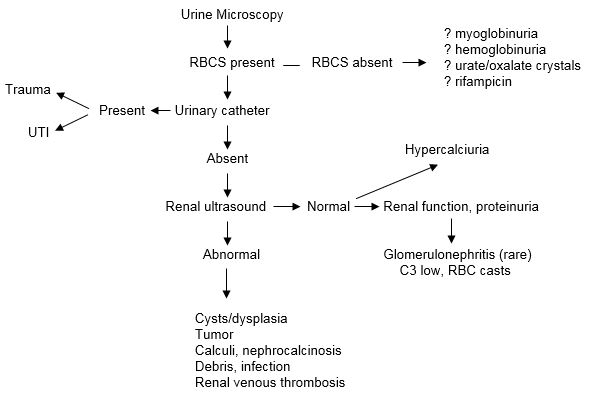
Diagnosis, investigation and management of urinary tract infection in the newborn.
A. Definition: Urine culture > 10 5 organisms/ml of a single colony count of bacteria. Incidence: 7% full term infants, 3% preterm infants; M: F = 5:1 (first month life)
B. Symptoms: fever, irritability, temperature instability, poor feeding, vomiting, metabolic acidosis, jaundice, lethargy, failure to thrive.
C. Predisposing Risk Factors:
- Urinary catheterization
- Vesicoureteric reflux
-
- Urinary tract malformations
- Urinary tract obstruction
- Neurogenic bladder
- Post circumcision
D. Organisms:
Escherichia coli, Klebsiella pneumonia, Proteus mirabilis, coliform bacteria, Pseudomonas aeruginosa, enterococci, staphylococci, streptococci Group B, Candida albicans (preterm infants)
E. Investigation:
-
- Urinalysis: positive leucocytes, nitrites
- Urine microscopy: fast, reliable and efficient.
- Urine culture: Bag specimens of urine are unreliable due to risk of contamination from stool or skin. Sterile bladder catheterization with a feeding tube or suprapubic aspiration, which in some units is performed under ultrasonic guidance.
- Sepsis work up: CBC, differential, BUN, Creatinine, Blood cultures.
- Radiographic investigations:
- Ultrasound:
- If reveals unilateral/bilateral small kidney, hydronephrosis, calculus, or large bladder →
- If normal AND male →
-
-
- VCUG:
- If normal, then repeat ultrasound at three months.
- If reveals dilated prostatic urethra or enlarged bladder → urgent Urology consultation.
- If reveals vesico-ureteric reflux → DMSA scan at 6 months.
-
F. Treatment:
Acute infection:
-
- Ampicillin and gentamicin for the first 48-72 hours until urine culture sensitivities available; then revise antibiotic selection according to culture results.
- Monitor therapeutic gentamicin levels regularly and adjust for renal impairment accordingly.
- The duration of therapy should be 7-10 days for an uncomplicated UTI. If meningitis confirmed, then therapy for a further 2-3 weeks may be warranted.
- Follow up urine specimen upon completion of the acute therapy.
Prophylaxis:
-
-
- Trimethoprim 2 mg/kg/day
- Recommended for infants at high risk for renal scarring:
- Obstructive uropathy
- Reflux with dilatation
- Neonates
- Delay in treatment
- Number of pyelonephritic attacks
- Uncommon bacteria (non- Escherichia coli)
-
Evaluation of nephrocalcinosis in the neonate
A. Definition: Deposition of calcium oxalate or calcium phosphate in the interstitium of the renal cortex/medulla.
B. Incidence: 5%of very low birth weight neonates 7.
C. Pathogenesis:
- Urinary supersaturation with stone promoters:
- Hypercalciuria (e.g. furosemide, vitamin D supplementation)
- Hyperphosphaturia (e.g. Fanconi syndrome)
- Hyperoxaluria
- Hyperuricosuria
- Hypercysteinuria
- Deficiency of stone inhibitors:
- Decreased urinary citrate excretion (e.g. renal tubular acidosis)
- Hypomagnesemia (e.g. neonatal Bartter’s disease)
- Hypopyrophosphaturia (e.g. hyperoxaluria).
D. Pertinent points on history and physical exam:
- History of antenatal renal anomalies (urinary stasis can predispose to crystal formation)
- Co-morbidity e.g. bronchopulmonary dysplasia, congenital heart defects, prematurity, rickets.
- Medications: furosemide, dexamethasone, vitamin D, methylxanthines.
- Family history of renal calculi, renal tubular acidosis.
- Nutrition: high protein intake, parenteral nutrition.
- History of urinary tract infection.
- Presence of sensorineural deafness- may be associated with neonatal Bartter’s syndrome (see below), distal RTA.
- Dysmorphic features e.g. ‘elfin facies’ of Williams’ syndrome may be associated with hypercalciuria.
E. Investigations:
Urine quantification of calcium, creatinine, urate, citrate, oxalate. Timed collections may be impractical. Spot urine may be used to screen for hypercalciuria by calculating Ca++:Creatinine ratio (mmol:mmol).
Premature infants (28-34 weeks): > 3.8 (7)
Term infants: > 2.24 (8)
F. Complications of nephrocalcinosis:
- Hypertension
- Urine concentrating defect
- Renal insufficiency
- Renal calculus with obstruction, infection, hematuria
Specific neonatal renal diseases
A. Neonatal Bartter’s syndrome:
- Hypokalaemia, hypochloraemic metabolic alkalosis secondary to high urinary losses of Na+, K+, Cl–; hyper-reninaemia, normal blood pressure
- Antenatal polyhydramnios
- Premature delivery
- Massive polyuria (12- 50 cc/kg/hr) with life-threatening dehydration
- Hypercalciuria and early onset nephrocalcinosis
- Mutations of NKCC2, ROMK, Barttin, ClC-Kb (Na/K/Cl transport in ascending limb of Henle’s loop)
- Barttin mutations are associated with sensorineural hearing loss
- Correct dehydration and electrolyte imbalance
- Indomethacin and potassium chloride after 4-6 weeks of life
B. Congenital Nephrotic Syndrome
- Fetal and neonatal proteinuria (>250mg/mmol urinary protein/creatinine)
- Placental weight > 25% birth weight, may be associated with flexion deformities.
- Premature and small for gestational age
- Elevated amniotic fluid alpha-fetoprotein
- Severe hypoalbuminemia (<10g/l) with oedema, ascites
- Mutations of proteins within the slit diaphragm of glomerular filtration barrier
- eg nephrin (NPHS1 > 90% Finnish type). Note: amniotic fluid alpha-fetoproteim will also be elevated in unaffected carriers.
- Secondary causes are typically infectious and include congenital CMV (TORCH), malaria, HIV, hepatitis B and C.
- Other secondary causes include congenital disorders of glycosylation, Immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, Mitochondrial Respiratory Chain deficiency, and LamB3 gene mutations (Herlitz junctional epidermolysis bullosa)
- Syndromic associations include Denys-Drash (WT1 mutations), Nail-Patella, Lowe’s, Frasier (WT1 mutations) and Pierson (‘microcoria’).
- Major complications are secondary to urinary protein losses and include infection, thrombosis, hypothyroidism and failure to thrive.
- High protein intake (4g/kg/day), high caloric intake (130kcal/kg/day)
- Daily albumin infusions (> 3g/kg/day) with furosemide therapy
- Anti-proteinuric medication eg ACE-I (Captopril), ARB (losartan)
- Treat hypothyroidism with thyroxine replacement therapy
- Unilateral nephrectomy may reduce protein losses sufficiently to decrease the frequency of albumin infusions and postpone the need for dialysis.
- Otherwise, bilateral nephrectomies at 7kg with peritoneal dialysis
- Renal transplant work up and transplantation at 10 kg
- Following renal transplantation, patient survival at 5 years is more than 90% and graft survival is approximately 80%.
C. Renal Tubular Acidosis
Definition: clinical syndrome of disordered acid-base balance in which the kidney fails to maintain a normal plasma bicarbonate concentration in the setting of anormal rate of acid production.
- The bicarbonate threshold is 21 mmol/L in the term infant, 18 mmol/L in the preterm infant and 14 mmol/L in the extremely premature infant, and it reaches adult levels of 24–26 mmol/L only after the first year
- Nonetheless, effective urinary acidification is usually acquired by the age of 1 month even in premature infants and this is independent of the gestational age at birth
Figure 4.
Diagnostic Algorithm For Renal Tubular Acidosis
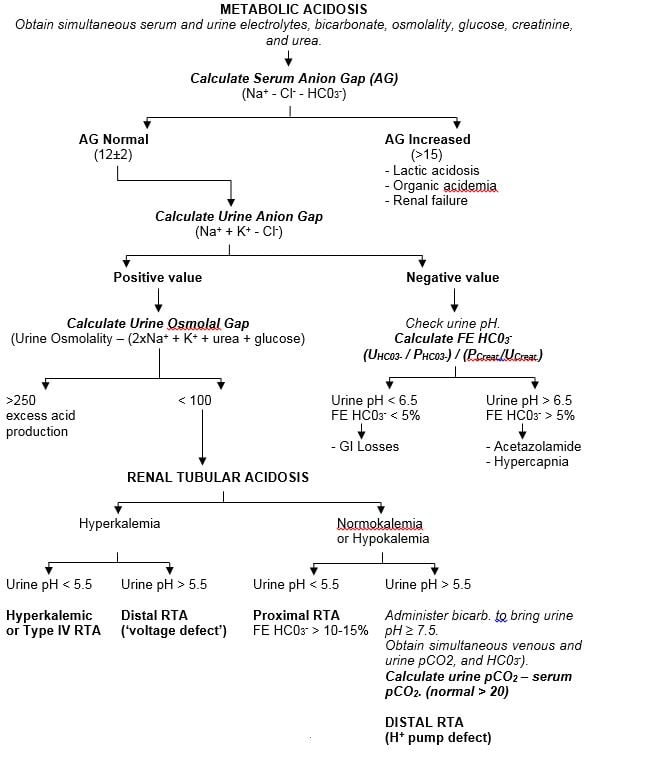
| Hyperkalemic or Type IV RTA | Distal RTA (‘voltage defect’) | Proximal RTA
FE HC03- > 10-15% |
Administer bicarb. to bring urine pH ≥ 7.5. Obtain simultaneous venous and urine pCO2, and HC03-).Calculate urine pCO2 – serum pCO2. (normal > 20)DISTAL RTA(H+ pump defect) |
D. Neonatal Hypertension
- Definition: BP > 95Th Centile for age
Table 10.
50th and 95th percentile values for systolic (SBP) and diastolic (DBP) blood pressure according to gestational age.
| BP | 24 wk | 28 wk | 32 wk | 36 wk | 40 wk | 44 wk |
|---|---|---|---|---|---|---|
| 95th SBP | 58 | 65 | 70 | 80 | 80 | 90 |
| 50th SBP | 40 | 45 | 50 | 50 | 55 | 75 |
| 95th DBP | 30 | 35 | 40 | 40 | 45 | 60 |
| 50th DBP | 15 | 25 | 30 | 30 | 35 | 45 |
Table 11.
Common Causes of Neonatal Hypertension and Relevant Investigations.
| Etiology | Investigations |
|---|---|
Renovascular
Renal parenchymal disease
|
Urinalysis (blood? Protein?)
BUN, Creatinine Renal USS with Dopplers Plasma renin, aldosterone DTPA/MAG3 VCUG |
Pulmonary
|
CXR |
Cardiac
|
Echocardiogram |
Endocrine, Metabolic
|
Cortisol, aldosterone, renin TSH, free t4 Calcium |
Medications
|
|
Neoplasia
|
Abdominal/pelvic Ultrasound Urine VMA, HVA |
Neurologic
|
Head ultrasound EEG |
Miscellaneous
|
|
Distal RTA may be associated with hypercalciuria and nephrocalcinosis
*Dose interval adjustment in renal impairment
# Dose interval adjustment in renal impairment
Where possible, treat the underlying cause Useful diagnostic tests include urinalysis (± culture), quantitative urinary protein/creatinine or albumin/creatinine ratios, CBC and platelet count, electrolytes, BUN, creatinine, calcium, chest x-ray, renal ultrasound with Doppler. Sometimes indicated tests include thyroid studies, urine VMA/HVA, plasma renin and aldosterone, cortisol, urinary 17-hydroxysteroids and 17-ketosteroids. and angiography Assess for secondary organ dysfunction eg, EKG, LVH on echocardiography. If steroid induced, consider diuretic.
|
|
References
- Kirpalani, H., Moore, A.M. and Perlman, M., 2007. Residents handbook of neonatology. PMPH-USA
- Rudd PT, Hughes EA, Placzek MM, Hodes DT. Reference ranges for plasma creatinine during the first month of life. Arch Dis Child.1983;58(3):212-5.
- Holtbach U, Aperia AC. Molecular determinants of sodium and water balance during early human development. Semin Neonatol. 2003; 8: 291-9.
- Agras PI, Tarcan A, Baskin E, Cengiz N, Gurakan B, Saatchi U. Acute renal failure in the neonatal period. Renal Failure. 2004;26 (3): 305-9.
- Cohen HL, Cooper J, Eisenberg P, et al. Normal length of fetal kidneys: sonographic study in 397 obstetric patients. AJR Am J Roentgenol. 1991;157(3):545-8.
- Wiesel A, Queisser-Luft A, Clementi M, Bianca S, Stoll C. Prenatal detection of congenital renal malformations by fetal ultrasonographic examination: an analysis of 709,030 births in 12 European countries. Eur J Med Genet. 2005;48(2):131-44.
- Clarren S. Malformations of the renal system. In: Holliday M, Barratt T, Avner E, eds. Pediatric Nephrology. 3rd ed. Philadelphia, PA: Williams & Wilkins; 1994:491-514.
- Hein G, Richter D, Manz F, Weitzel D, Kalhoff H. Development of nephrocalcinosis in very low birth weight infants. Ped Nephrol 2004;19 (6): 616-20.
- Aladangady N, Coen PG, White MP, Rae MD, Beattie TJ. Urinary excretion of calcium and phosphate in preterm infants. Pediatr Nephrol. 2004;19(11):1225-31.
- Sargent JD, Stukel TA, Kresel J, Klein RZ. Normal values for random urinary calcium to creatinine ratios in infancy. J Pediatr. 1993;123(3):393-7.