Neurology
Dr Amr El Shahed, MD, Dr Jarred Garfinkle, MD and Dr Aideen Moore, MD, FRCPC, MRCPI, MHSc
The material presented here was first published in the Residents’ Handbook of Neonatology, 3rd edition, and is reproduced here with permission from PMPH USA, Ltd. of New Haven, Connecticut and Cary, North Carolina.
- Newborn infants have a restricted repertoire of neurological signs and symptoms. Even with severe underlying pathological insults, newborns often exhibit only subtle findings.
- Injury to the newborn brain can have a lifelong, important impact on the child (and eventual adult) and family
- This chapter covers neonatal seizures, neonatal encephalopathy and hypoxic ischemic encephalopathy (HIE), hemorrhagic and ischemic intracranial lesions including trauma, brain injury in the preterm newborn and the newborn presenting with hypotonia.
Neonatal Seizures
- A seizure is defined clinically as a paroxysmal alteration in any neurologic function and is typically accompanied by epileptic activity identifiable on an EEG or amplitude-integrated EEG (aEEG).
- Seizures in newborns are rarely idiopathic and usually symptomatic of an underlying acute brain injury.
- Seizures may in and of themselves contribute to brain injury, neurodevelopmental impairment, and epileptogenesis. As such, prompt treatment is warranted.
Etiology
- The relative frequencies of the etiologies vary depending on the gestational age.
- The timing of onset can offer a clue as to the etiology.
- Hypoxic-ischemic brain injury is the most common etiology of neonatal seizures in term neonates, followed by arterial and venous stroke.
Neonatal Seizure Etiologies in Relation to Onset and Relative Frequency
| Relative frequency | |||
| Etiology | Typical age at onset | Preterm | Term |
| Hypoxic ischemic encephalopathy | < 24 hours | + | ++ |
| Intracranial hemorrhage (epidural, subdural, subarachnoid, parenchymal, and/or intraventricular) | < 3 days | ++ | + |
| Stroke (arterial or venous) | 1-3 days | + | ++ |
| Meningitis/encephalitis | Variable | + | + |
| Hypoglycemia | < 2 days | + | + |
| Hypocalcemia | Variable | + | + |
| Other metabolic conditions | <3 days | + | |
| Cerebral malformation | Variable | + | |
| Benign familial neonatal seizures | <7 days | + | |
| Other genetic epilepsies (e.g., channelopathies) | Variable | + | |
- Neonatal epilepsy syndromes, which encompass channelopathies and specific metabolic deficiencies, can range from benign to severe.
- Benign familial neonatal epilepsy is an autosomal dominant condition for which several gene loci which encode for voltage-gated channels (KCNQ2, KCNQ3, and SCN2A) have been identified.
- KCNQ2 mutations can also lead to severe epileptic encephalopathies.
Diagnosis, semiology, and differential diagnosis
- Seizure manifestations in newborns are not typically well-organized, tonic-clonic seizures due to the immaturity of synaptic connections
Clinical seizure classification
| Clinical seizure | Manifestations and key points |
| Subtle | Eye deviation, blinking, fixed stare
Repetitive mouth and tongue movements Apnea, other autonomic phenomena Bicycling, other rhythmic limb movements |
| Clonic: focal or multifocal | Rhythmic movements of muscle groups
Often represents a focal pathology |
| Tonic: focal or generalized | Sustained flexion or extension of muscle groups |
| Multifocal: focal, multifocal, or generalized | Synchronous flexion jerks
Must be distinguished from nonepileptic myoclonus |
From Volpe’s Neurology of the Newborn, 6th Edition.
- Seizures in the newborn are often clinically silent and only detected on EEG or aEEG; these seizures are referred to as subclinical seizures. Electroclinical seizures (clinical seizures with an electrographic correlate) often become subclinical after treatment with antiepileptic agents.
- Clinical seizures without electrographic correlate on EEG are rare. For example, seizures emanating from the temporal lobe, which may cause isolated apnea, may not be detected on standard EEG.
- EEG monitoring to identify seizures is essential to avoid missing (and undertreating) seizures and to avoid overdiagnosing (and overtreating) seizures.
- If continuous EEG is not available, then continuous amplitude-integrated EEG (aEEG) can be applied. aEEG is a simplified trend monitor that displays one or two channels of time-compressed, processed EEG and its interpretation is enhanced by concurrent display of the raw single or multichannel EEG.
- Compared to EEG, aEEG is less sensitivity. In particular, it might miss brief seizures.
- Seizures must be distinguished from clonus and jitteriness
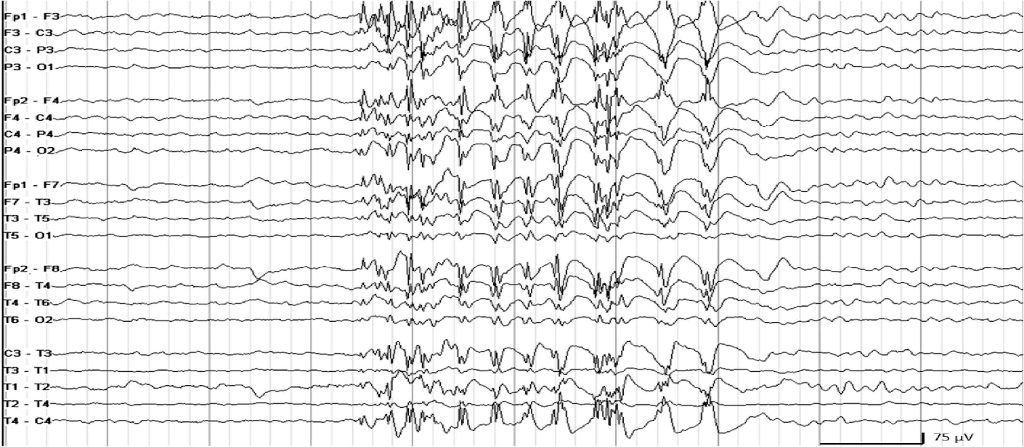
An EEG generally contains between 16-24 recording electrodes
Investigations and management
- Neonatal seizures require urgent investigation for acute-symptomatic etiologies and management because prolonged and recurrent seizures are likely independently associated with brain injury.
- Investigations should occur concurrent with seizure management.
- Initial investigations should include: neurologic examination, bedside glucose, electrolyte panel, full sepsis workup (blood and cerebrospinal fluid), and neuroimaging as available institutionally.
- Treatment with antiseizure medications should be initiated once electrographic seizures are confirmed or if the clinical suspicion for seizures is sufficiently high in the absence of electrographic corroboration.
- We recommend Lorazepam as first line management due to its rapid onset and offset of action. After two doses of Lorazepam, we recommend a loading dose of Phenobarbital.
- Lorazepam, a benzodiazepine, and phenobarbital, a barbiturate, are GABAergic medications. Side effects include respiratory depression and low blood pressure.
- Levetiracetam binds to the synaptic vesicle protein SV2A and modulates synaptic transmission.
- Phenytoin blocks voltage-dependent neuronal sodium channels. It is particularly effective in many channelopathies.
- In the context of refractory seizures of unknown etiology, treatable metabolic epileptic encephalopathies should be considered, and the appropriate therapy instituted pending diagnosis.

- Seizures should be treated promptly because delays to treatment may make them harder to abort.
- Lorazepam is recommended as initial therapy for acute clinical neonatal seizures because of its ease of administration and rapid onset of action, when compared to Phenobarbital.
- A Phenobarbital load should be administered over 10 minutes (2mg/kg/min) by IV slow push or infusion, with close monitoring for potential bradycardia and hypotension.
- Patients receiving anticonvulsants must have continuous cardiorespiratory monitoring.
- Additional treatment options include Levetiracetam (Keppra) and, if unavailable, Fosphenytoin (Cerebyx).
- If seizures recur following a seizure-free interval, clinical judgment should be used to decide at which step in the guideline to resume therapy.
- Pyridoxine dependent epilepsy should be considered in all newborns with unexplained or refractory seizures.
- Consultation with Biochemical Diseases service should be initiated in any child with medically refractory seizures requiring a midazolam infusion.
Prognosis and later epilepsy
- The major determinant of neuroprognosis following neonatal seizures is the underlying etiology and degree of brain injury.
- In most acute symptomatic seizures, the seizures remit within a few days of life and continuation of antieplileptic medications after discharge is not indicated.
- In cases that require multiple antiepileptic medications or those that have a period of status epilepticus, continuation of antiepeileptic medications (e.g., Phenobarbital) after discharge may be warranted due to the high risk of seizure recurrence.
Neonatal Encephalopathies:
Neonatal encephalopathy is a clinical syndrome of disturbed neurological function that presents early in life, with an incidence of approximately 1/1000 to 6/1000 live births.
Hypoxic-ischemic Encephalopathy
Hypoxic-ischemic encephalopathy (HIE) accounts for a significant proportion of encephalopathic newborns. Despite advances in perinatal care, moderate-to-severe acute perinatal HIE in late preterm and term infants remains an important cause of mortality, acute neurological injury and subsequent long-term neurodevelopmental disability. Impaired cerebral blood flow (ischemia), in the setting of insufficient oxygenation (hypoxia), is the main mechanism causing brain injury.
Pathophysiology:
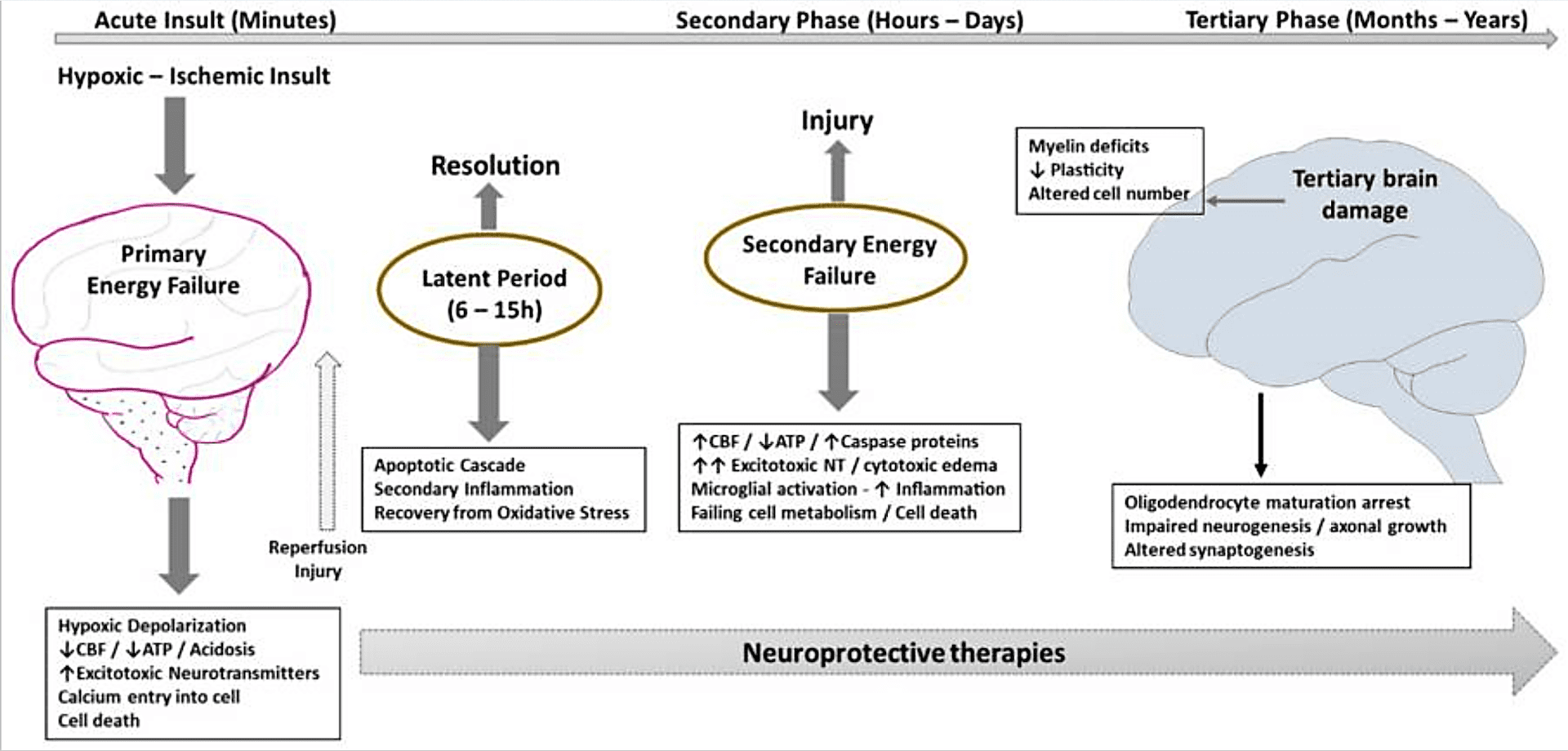
At the cellular level, hypoxia-ischemia results in two phases of energy failure:
- The primary phase follows the reduction in blood flow and oxygen supply, with a fall in adenosine triphosphate (ATP), failure of the sodium (Na+)/potassium (K+) pump, depolarization of cells, lactic acidosis, release of excitatory amino acids, calcium entry into the cell and, when severe, cell necrosis.
- Following resuscitation and reperfusion, there is a latent period of 1 to 6 hours where the impairment of cerebral oxidative metabolism can at least partially recover, before irreversible failure of mitochondrial function. This latent phase is the therapeutic window for neuroprotective intervention.
Risk Factors:
Intrapartum:
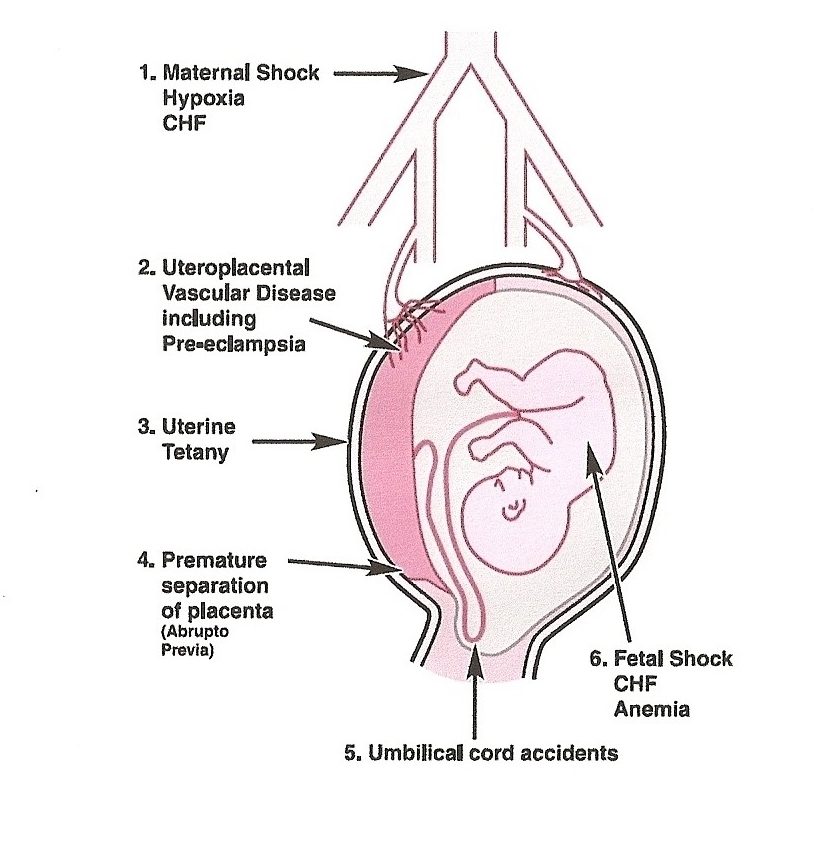
- The principal intrapartum events leading to hypoxic-ischemic fetal insults include acute placental or umbilical cord disturbances, such as abruptio placentae, cord prolapse, tight nuchal cord, prolonged labor with transverse arrest, difficult instrumental extractions, or rotational maneuvers, shoulder dystocia or uterine rupture.
- In addition to the history of sentinel event, features suggestive of acute asphyxia include:
-
- Sudden and sustained fetal bradycardia, or the absence of fetal heart rate variability with persistent late or persistent variable decelerations when the pattern was previously normal.
- Low Apgar scores <5 at 5 and 10 minutes, need for prolonged resuscitation.
- Significant cord gas acidemia PH ≤ 7, base deficit ≥ 10.
- Evidence of multiorgan dysfunction (oliguria, rising serum creatinine concentration after birth, rising AST and ALT, high troponin level with myocardial dysfunction).
Antepartum:
- Hypoxic-ischemic injury certainly can occur in the antepartum period (e.g., secondary to maternal trauma, maternal hypotension, uterine hemorrhage), this injury cumulatively accounts for only a small proportion of neonatal HIE.
- Antepartum factors may predispose some women to experience adverse intrapartum episodes during the stresses of labor and delivery, especially through threats to placental flow. Such factors include maternal diabetes, preeclampsia, placental vasculopathy, intrauterine growth retardation, and twin gestation.
- In addition to the history, features suggestive of chronic asphyxia include:
-
-
- Episode of loss of fetal movements for 12 or more hours
- Oligohydramnios
- Intrauterine growth restriction (IUGR)
- Loss of fetal heart rate variability or persistent nonreactive heart rate tracing
- Meconium staining of fetal membranes, placenta, and neonatal nails and skin
- Often these babies do not require extensive resuscitation, with Apgar scores ≥ 5 and mild academia
-
In a recent study, neonates with encephalopathy had more frequent antepartum (74% versus 18%) and intrapartum (67% versus 19%) risk factors than controls. Incidence of risk factors was 22% intrapartum, 26% antepartum, 44% antepartum & intrapartum and unknown in 8% of cases.
Clinical manifestation and diagnosis:
- Documented evidence of acute sentinel event.
- Evidence of intrapartum hypoxia: at least one of:
a) Apgar score of ≤5 at 10 minutes;
b) mechanical ventilation or resuscitation within first 10 minutes of life
c) Cord pH < 7.00 (venous or arterial), or an infant arterial pH < 7.00 or base deficit ≥ 12 within 60 minutes of birth. - Manifestations of cerebral dysfunction with/without multi-organ involvement:
| System | Clinical manifestation and complication |
| Nervous | hypotonia, hypo/hyper-reflexia, cranial nerve palsies, seizures, coma |
| Cardiovascular | myocardial ischemia, tricuspid insufficiency, ventricular dysfunction, heart failure |
| Respiratory | hypoxia, respiratory acidosis, PPHN, surfactant dysfunction, meconium aspiration, pulmonary hemorrhage/edema |
| Gastro-intestinal | feeding intolerance, NEC, hepatic dysfunction, liver failure |
| Renal | oliguria (acute tubular necrosis, SIADH, renal failure) |
| Metabolic | lactic acidosis, hypoglycemia, hyponatremia, hypocalcemia |
| Hematologic | anemia, neutropenia, thrombocytopenia, DIC |
Differential Diagnosis:
Often infants who have NE have perinatal antecedents, onset of symptoms, and a clinical course that are consistent with and typical of HIE. However, in some cases investigations are necessary to look for non–hypoxic-ischemic causes of NE such as:
| History and clinical features | Investigations | |
| Bacterial Meningitis | maternal fever, infection, chorioamnionitis | lumbar puncture may not be essential in lumbar puncture may not be essential in a typical case of HIE |
| Viral encephalopathy | maternal infection, neonatal skin lesion | blood and CSF PCR (empiric antiviral therapy is not indicated in a typical case of HIE) |
| Hypoglycemia | maternal diabetes | blood glucose, electrolytes and gas |
| Stroke | birth trauma, unilateral seizures | unilateral electrical seizures brain MRI |
| Metabolic encephalopathy (eg, mitochondrial and peroxisomal disorders, Molybdenum cofactor deficiency) | family history of IEM, abnormal facies | persistent lactic acidosis, abnormal VLCFA, elevated urinary sulfite and S-sulfocysteine |
| Intracranial hemorrhages | Birth trauma, coagulopathy | Anemia, coagulopathy, HUS, brain MRI |
Management:
- Supportive measures as indicated including:
- Respiratory support: noninvasive eg: CPAP or invasive mechanical ventilation (suppressed conscious level, apnea, respiratory depression secondary to AED).
- Cardiovascular support:
a) volume expander (only in case of hypovolemia eg fetal or placental hemorrhage),
b) inotropic support (in context of right ventricular dysfunction, PPHN and hypotension; carful choice of drug:
– Dopamine (improve cardiac contractility, enhance renal perfusion, can cause pulmonary vasoconstriction)
– Dobutamine (improve cardiac contractility, can cause tachycardia and low blood pressure)
– Vasopressin (support SVR, decrease PVR, can cause water retention and hyponatremia)
– Adrenaline (improve cardiac contractility and SVR, can cause hyperglycemia and PIV burn)
– Milrinone (reduce PVR, better avoided because of systemic VD effect). - Hematological: PRBC, platelet or fresh frozen plasma and vit K as indicated.
- Fluid management and nutrition: fluid restriction (in context of brain edema and acute renal injury), diuretics (to force diuresis, should be used with caution due to risk of hypovolemia in context of myocardial dysfunction). NGT/oral feeding usually starts after rewarming (to ensure good gut perfusion).
2. Treatment of seizures (see Page–)
3. Therapeutic Hypothermia:
Indication (The Hospital for Sick Children Criteria for cooling):
- Must fulfill all 4 criteria:
1) Gestational Age greater than or equal to 35 weeks
2) Less than 6 hours post-delivery or hypoxic arrest
3) Evidence of intrapartum hypoxia
a. Cord gas / within an hour of birth with pH <7.0 OR base deficit ≥-16
OR
b. pH 7.01-7.15 OR base deficit –10 – -15.9 with evidence of acute perinatal even that may result in HIE (e.g abruptio placentae, cord prolapse, uterine rupture, etc)
AND Apgar score 5 or less at 10 minutes, need for mechanical ventilation or resuscitation at 10 minutes.
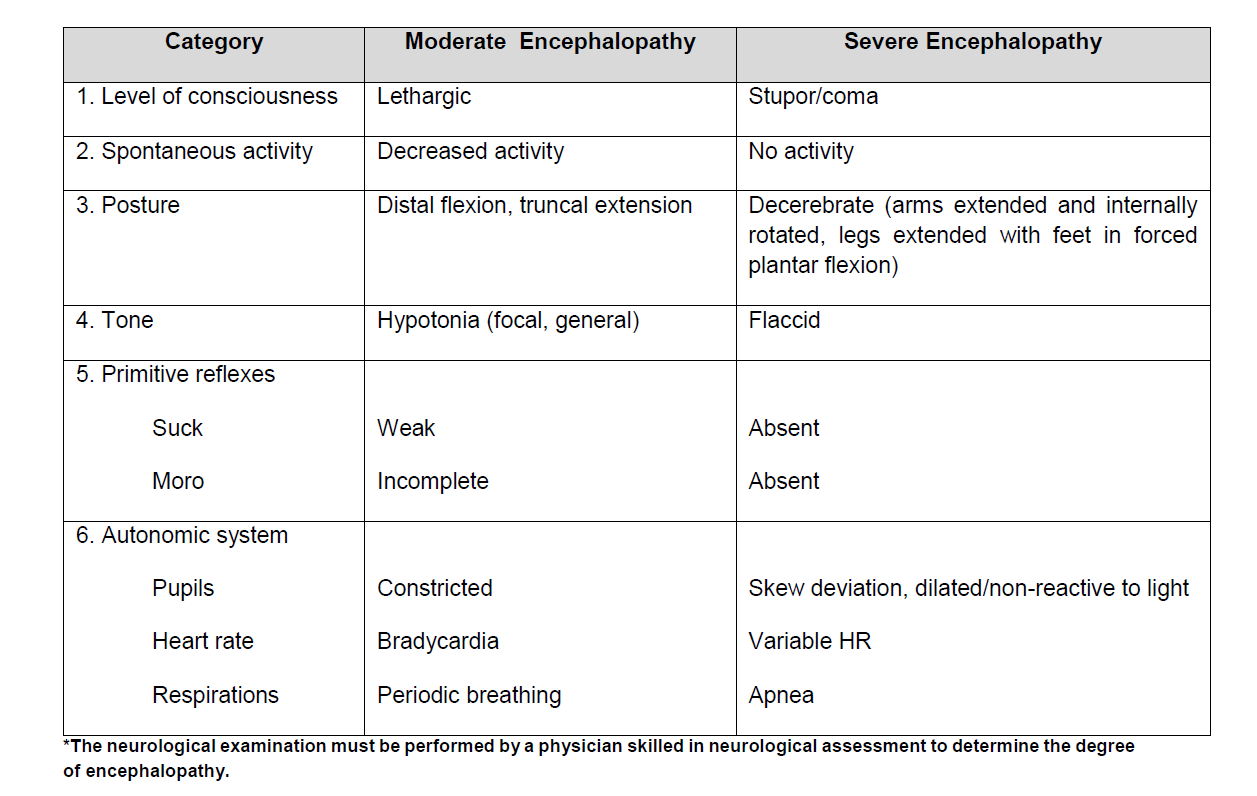
4) Signs of moderate or severe encephalopathy defined as the presence of 3 or more of the items in the 6 categories below OR presence of seizures:
- Earlier cooling produces more neuroprotective effect based on animal and some clinical studies, however, it is more important during first few hours after life to focus on stabilization of respiratory and hemodynamic condition of the baby.
- There is no evidence to support that initiation of Th after 6 hours reduces secondary brain injury.
- Serial structured neurological assessment during first 6 hours is important to properly identify neonates who are eligible to receive TH.
- There is no enough evidence about safety and efficacy of TH in treatment of preterm < 35 weeks of gestation with HIE.
Methods of cooling:
- The goal of TH is to lower temp of the vulnerable deep brain structures, the basal ganglia to 32-34C.
- Selective head cooling with mild systemic hypothermia (Rectal temp 34-35C)
- Systemic hypothermia (core temp 33-34C)
- With cooling blanket
- With cool packs
- Servo-controlled fan
Neuroprotective effect of TH:
- Reduces cerebral metabolism, prevents edema and loss of membrane potential
- Decreases energy utilization
- Reduces/suppresses cytotoxic AA accumulation and NO
- Inhibits inflammatory cascade
- Suppresses free radical activity and lipid peroxidation
- Attenuates secondary energy failure
- Inhibits apoptosis and necrosis
- Reduces extent of brain injury
Evidence support use of TH:
- Five major randomized controlled trials (CoolCap Gluckman. Lancet 2005, NICHD, Shankaran. NEJM 2005, TOBY Azzopardi. NEJM 2009, Neo-nEuro Simbruner. Pediatrics2010 and ICE Jacobs. Arch Ped Adol Med 2011) showed clinical benefit of cooling.
- Systematic metanalysis showed that Trials of TH demonstrated a 15% reduction in the rates of mortality and moderate to severe neurodevelopmental disabilities.
- Neuroprotective effect of TH in neonates with chronic hypoxic/ischemic insult is controversial.
Brain monitoring and prognostic modalities:
- Amplitude integrated electroencephalogram (aEEG):
- Simple bedside 3 or 4 channels tool to record electrographic brain activity.
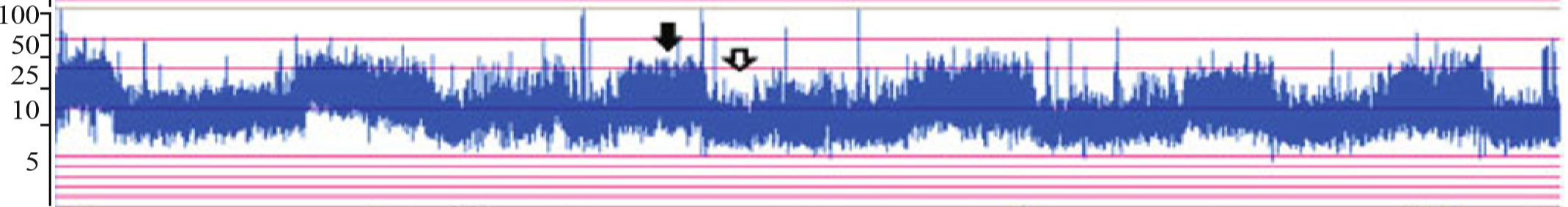
- Normal electrical background activity of full-term neonate has lower margin of > 5 μV and upper margin > 10 μV with sleep and awake cycle (black and white arrows).
- Changes of background activity correlates with degree of encephalopathy.
- The duration of aEEG abnormalities correlates with neurological outcome: in mild cases, there is a rapid recovery of aEEG within 6 to 12 hours, and the prognosis is excellent.
- Abnormal aEEG (Burst suppression or worse trace), persisting beyond 48 hours had a poor outcome, despite cooling.
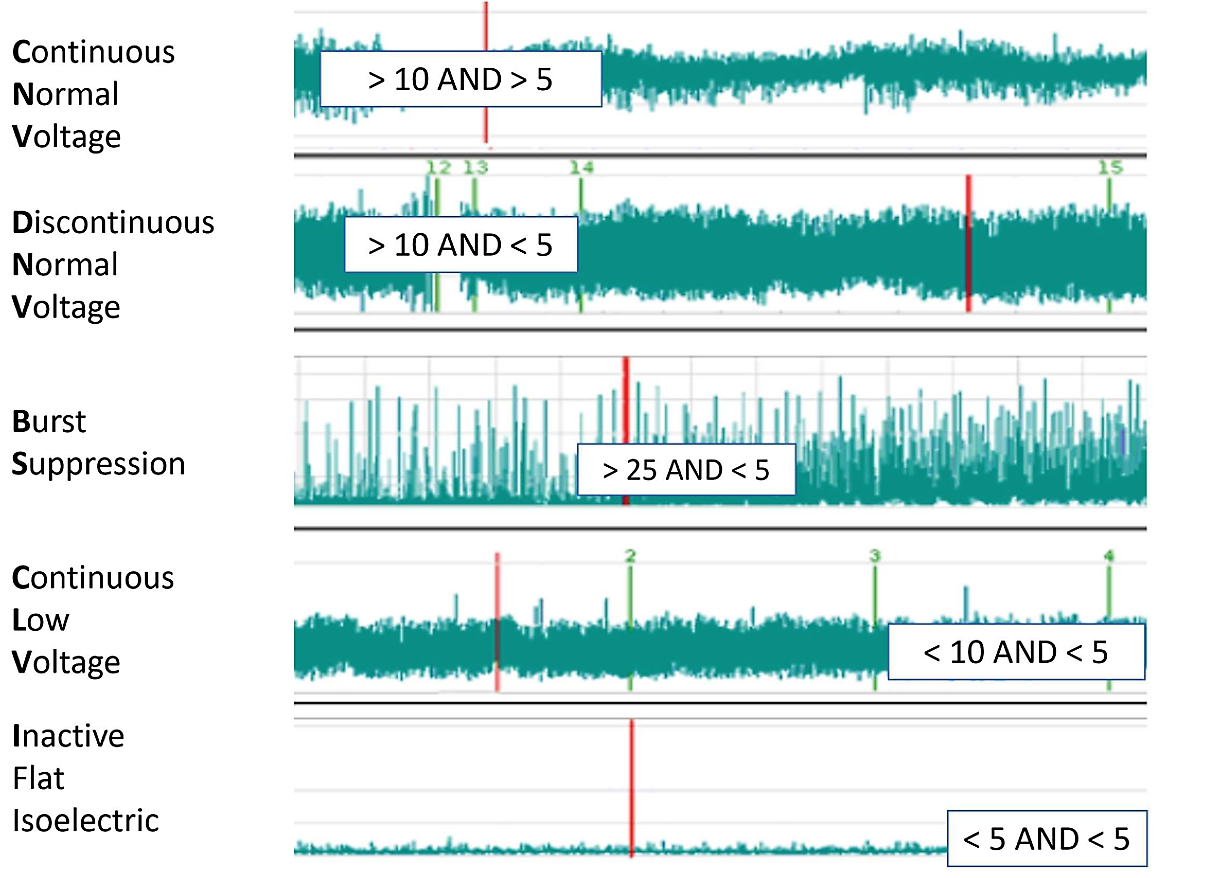
2. Continuous video electroencephalogram:
- Indicated in presence of suspected clinical or aEEG seizures or presence of abnormal suppressed aEEG background (increased risk of seizures).
- Features of encephalopathy include reduced amplitude and frequency, increased inter-burst intervals (IBI) and the presence of multifocal or focal sharp waves.
- Infants with continuous or very repetitive seizures usually have a severely suppressed interictal background EEG/aEEG and a poor prognosis, despite cooling.
3. Near Infra-red Spectroscopy (NIRS):
- Measures regional oxygen saturation (rSO2) and reflects the amount of oxygen extracted by brain tissue from the vascular compartment and in this manner describes the balance between the cerebral oxygen delivery and the cerebral oxygen consumption.
- Studies showed that newborns with severe HIE had high cerebral rSO2 and extracted less oxygen (likely lower oxygen demand or utilization)when comparing to those with moderate HIE. High cerebral rSO2 correlates with poor outcome.
4. Evoked potentials:
- Evoked potentials are the electrical response to a provided sensory stimulation and include visual evoked potentials (VEP), brainstem auditory evoked response (BAER), and somatosensory evoked potentials (SSEP).
- Persistent absence of VEP in repeated tests indicates poor prognosis.
- Normal SEP indicates good prognosis.
- Increasing use of advanced neuroimaging, the reliance on evoked potentials has significantly declined in the last few years.
5. Head US:
- Indicated in first day of life to role out intracranial hemorrhage, hydrocephalus and cranial malformation.
6. Brain MRI
- Diffusion weight imaging (DWI) may demonstrate cytotoxic edema (due to hypoxic brain injury) in acute phase, seen as diffusion restriction before the signal intensity changes are evident on conventional T1- or T2-weighted images.
- The limitation of DWI is that it may give false negative result if performed within first 24 h of HI injury. DWI changes can be typically seen for only 10–12 days after tissue death and pseudo-normalization occurs thereafter.
- Conventional MRI (T1 and T2) is less sensitive than newer imaging techniques like DWI and Magnetic resonant spectroscopy (MRS) in diagnosing acute brain injury.
- Elevated lactate/creatine ratio in MRS on day 1 of life is a predictor of adverse neurological outcome, whereas absence of lactate predicts a normal outcome.
Pattern of brain injury:
a. parasagittal watershed zone infarcts between anterior/MCA and middle/posterior cerebral artery and subcortical white matter injury, mostly correlates mild to moderate hypoperfusion injury or chronic HI injury. (fig, a)
b. Severe HI injury results in injury to metabolically active tissues such as ventrolateral thalami, posterior putamina, hippocampi, brainstem, corticospinal tracts, and perirolandic cortex. Basal ganglia (BG) injury is more common than parasagittal pattern and carries the worst prognosis.(fig, b)
c. Mixed global injury in more severe diffuse brain injury (fig, c)
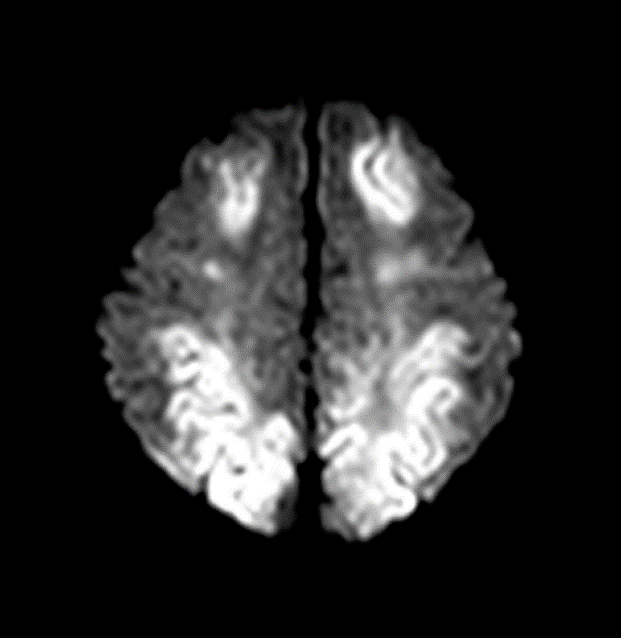
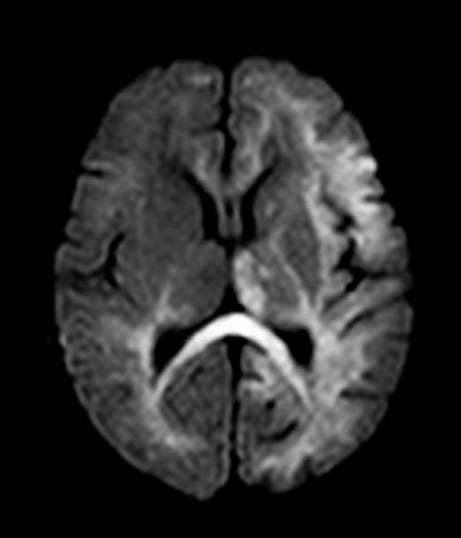
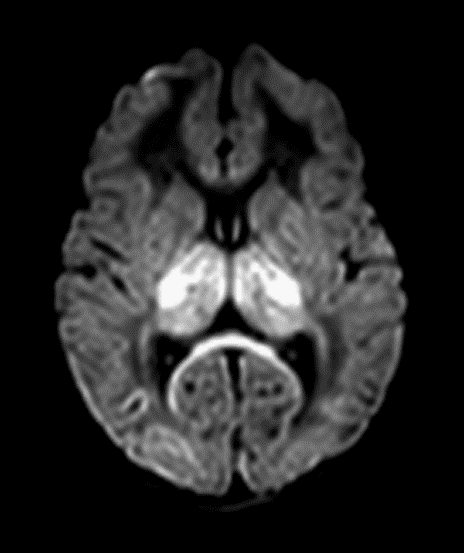
| Predictors of outcome | Pros | Cons |
| Acid base (PH and BD) | Earliest and most sensitive signs of fetal distress. | PPV for significant
encephalopathy and abnormal outcome is low |
| Lactate | Better reflects metabolic mechanism | No advantage over pH |
| Apgar score | Quick assessment of neonatal condition at birth, noninvasive | High inter-observer variability, poor predictor of long-term outcome |
| Clinical examination | Non-invasive, good to track changes in clinical state as
injury evolves, predictive at discharge |
Requires clinical experience, affected by intubation and
medications and hypothermia, poor predictor of long-term outcome |
| HUS | Safe, available at bedside, can be repeated | Predictive accuracy is poor, with a high false-positive rate |
| EEG/aEEG | “Gold standard”, early predictive value if normal, value
of subclinical seizure detection, non-invasive |
Requires resources, equipment to apply, clinical expertise to
interpret |
| MRI/MRS | Specific patterns of injury aid prognosis, early changes apparent. Highest predictive value in compare to other prognostic tests | Requires specialist equipment, requires transfer of sick infant
to MRI machine/department, requires infant to remain still for prolonged periods |
Long-Term Complications of HIE
When cerebral edema resolves, the brain stem may regain control and spontaneous respirations are established. Infant may survive with one or more of the following long-term neurodevelopmental deficits.
- Brain damage
a. Spastic quadriplegia, often with microcephaly
b. “Mixed” spastic quadriplegia and athetosis
c. Dystonic, dyskinetic, or athetotic cerebral palsy
d. Cortical blindness (visual cortical impairment)
e. Epilepsy
f. Sensorineural hearing loss
g. Cognitive deficits and behavioral problems
- Other permanent organ damage (rare)
a. Permanent kidney damage (hypertension, end stage renal disease) (rare)
b. Myocardial and heart valve damage (rarely clinically significant)
c. Liver damage (cirrhosis) (extremely rare)
Emerging Future Neuroprotective drugs:
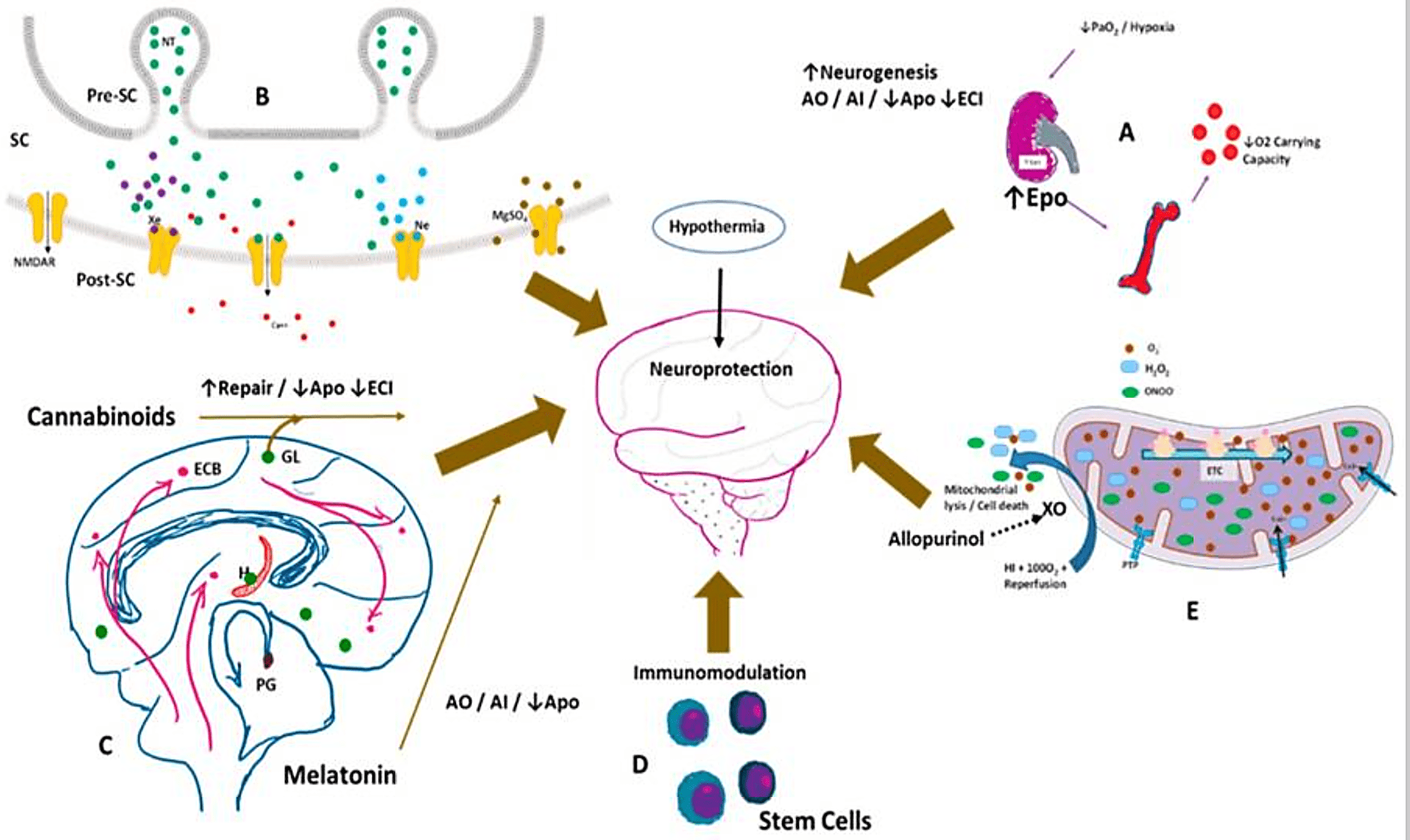
Potential neuroprotective therapies in the management of hypoxic-ischemic encephalopathy. Hypothermia is currently the standard of care in the management of moderate to severe HIE in infants. (A) Hypoxia stimulates erythropoietin (Epo) production by the kidneys, increasing RBC production in the bone marrow, thereby increasing O2 carryign capacity. Epo favors neurogenesis; is an antioxidant (AO), anti-inflammatory (AI), decreases apoptosis (Apo) and excitotoxic cell injury (ECI); (B) Xenon (Xe—purple dots), neon (Ne—teal dots), and magnesium sulphate (Mgso4—brown dots) antagonize the N-methyl-D-aspartate (NMDA) mediated excitotoxicity via the NMDA receptor (NMDAR), decreasing calcium entry into the cell. SC—synaptic cleft, Pre-SC—presynaptic cleft, Post-SC—postsynaptic cleft; (C) Endogenous cannabinoids (ECBs) and melatonin are neuroprotective. Spingolipids such as gangliosides (GL) also protect against apoptotic injury; (D) Umbilical cord blood derived stem cells and mesenchymal stem cells modulate the immune system and affect long-term outcomes; (E) Allopurinol by inhibiting the enzyme xanthine oxidase (XO), decreases reactive oxygen species, mitochondrial lysis, and cell death. O2—superoxide, H2O2—hydrogen peroxide, ONOO—peroxynitrite, ETC—electron transfer chain; PTP—permeability transition pore of inner mitochondrial membrane.
Brain Injury in the Preterm Newborn
- Two major upstream mechanisms are responsible for interrupting brain development in the rapidly developing preterm brain: ischemia and infection/inflammation
- Among contemporary cohorts of children born very preterm, 5%-10% have major motor deficits, including cerebral palsy, and more than half have significant cognitive, behavioral, or sensory deficits
- Many of the “softer” neurodevelopmental challenges are likely linked to impairments in cerebral growth and disturbances in cerebral maturation rather than overt injuries
Intraventricular hemorrhage
- IVH originates in the subependymal germinal matrix.
- Cortical neuronal and glial cell precursors develop from the germinal matrix
- Involution of the germinal matrix occurs with advancing gestation, prior to which it is highly vascularized.
Pathogenesis
- The preterm brain’s arterioles lack autoregulation and exist in a pressure-passive state.
- The germinal matrix blood vessels lack a supporting basement membrane.
- IVH may occur in the setting of elevated venous pressure or an increase in fluctuations in cerebral blood flow, which may be caused by:
- respiratory distress;
- pneumothorax;
- asphyxia;
- left ventricular dysfunction;
- patent ductus arteriosus;
- hypotension;
- hypothermia; and
- hyperosmolarity
Timing, presentation, and grading
- The risk period for of IVH is highest in the first 3 or 4 days of life, with 50% detectable by 24 hours.
- The clinical presentation of IVH in the newborn depends on the extent of the hemorrhage but is usually asymptomatic.
- The classical grade IV hemorrhage arises from venous infarction of the periventricular white matter rather than from a direct extension of the IVH into the parenchyma.
- Hydrocephalus may occur subsequent to grade III or IV IVH.
- Symptoms of progressive hydrocephalus are a late marker of ventricular dilation, and include:
- increasing head circumference;
- a full anterior fontanel;
- separation of cranial sutures; and
- apneas, bradycardia, or hypertension.
Prevention
- Prenatal administration of steroids and maternal transfer to a tertiary level hospital are the best means of preventing IVH.
- Practice bundles that limit fluctuations in cerebral blood flow include:
- maintenance of PCO2 and metabolic status within a normal range;
- avoidance of excessive suctioning and handling; and
- avoidance of rapid volume infusions and infusions of sodium bicarbonate.
Management and prognosis
- Guidelines for head ultrasound vary and depends on the risk for IVH or IVH progression. Typically, one is done after the first 3 days of life and repeated after the first week in newborns at risk for IVH (e.g., ≤ 32 weeks).
- Serial head ultrasound examinations are necessary in the setting of significant IVH because many such neonates develop progressive ventricular dilatation.
- In the setting of ventricular dilation, diversion of cerebrospinal fluid before the onset of signs of high intracranial pressure is recommended.
- Most neonates with mild IVH are not a much higher risk for neurodevelopmental impairments.
- Neonates with Grade III IVH, and especially those with associated periventricular hemorrhagic infarction or posthemorrhagic ventricular dilatation, are at higher for poor neurodevelopmental outcome.
White matter injury
- White matter injury (WMI) is the commonest form of brain injury in preterm neonates.
- WMI is linked in experimental models and clinical studies to hypoxia-ischemia, infection, and inflammation.
- Periventricular leukomalacia is characterized by periventricular focal necrosis with subsequent cystic formation.
- The large necrotic lesions of periventricular leukomalacia have become uncommon in contemporary cohorts of preterm neonates and diffuse punctate WMI is the predominant lesion in most preterm neonates.
- On diagnostic MRI scans, WMI appears as areas of abnormal signal intensity with a characteristic topology, with most lesions occurring in the central periventricular region.
Neonatal Brain Infection
Bacterial meningitis
- Neonatal meningitis is the most frequent bacterial infection of the central nervous system.
- Early recognition and appropriate therapy of bacterial meningitis are important because of its fulminant natural course.
Pathogenesis and Microbiology
- Early-onset meningitis (within the first few days of life) is likely derived from an infected birth canal and is usually accompanied by systemic illness.
- The most common bacterial causes of early-onset meningitis are Group B streptococcus and Escherichia coli.
- Late-onset disease usually presents with isolated meningitis and neurologic signs such as lethargy, seizures, cranial neuropathies, and hydrocephalus.
- Vasculitis from meningitis may result in occlusion of arteries and veins with thrombosis
- Abscess formation from tissue necrosis with may complicate meningitis from certain gram-negative species, such as Enterobacter and Serratia.
- Hydrocephalus can be a late complication due to obstruction of CSF flow.
Diagnosis and Treatment
- Cerebrospinal fluid analysis is the most important diagnostic test and meningitis is characterized by an elevated CSF WBC count predominantly comprising polymorphonuclear leukocytes, an elevated protein concentration, and a depressed glucose concentration.
- In preterm neonates, normal values for CSF WBCs have not been definitively established.
- Bacterial PCR techniques directed against the 16S ribosomal RNA subunit have recently been used to identify pathogens in ambiguous cases.
- Head imaging (ultrasound and MRI) is helpful in defining the complications of meningitis, such as empyema, infarction, and hydrocephalus.
- Empiric antibiotic treatment with Ampicillin and cefotaxime is indicated in the neonate with suspected bacterial meningitis.
Neonatal herpes
- Neonatal herpes simplex infection is usually acquired soon before or during passage through an infected birth canal in most cases.
- The risk for infection is highest when the mother has a primary HSV infection, which is often asymptomatic, and considerably lower with reactivated infection.
- Neonatal herpes infection is always symptomatic and may present as (1) localized oral, cutaneous, or ophthalmic disease; (2) localized disease of the CNS; or (3) disseminated disease with liver injury, disseminated intravascular coagulation, and renal failure, with or without meningoencephalitis.
- Brain disease if characterized by extensive, often hemorrhagic, meningoencephalitis.
- Studies of the CSF usually are consistent with viral meningoencephalitis, and often show red blood cells.
- Diagnosis is most readily established using serum and CSF PCR for HSV.
- MRI, especially diffusion-weighted MRI, is useful for delineating the extent and severity of brain injury but there is no definitive pattern of brain injury.
- A 21-day course of acyclovir should be started empirically in term neonates with suspected HSV encephalitis and has been shown to improve mortality and neurologic outcomes.
- Additional suppressive therapy with oral acyclovir for 6 months immediately following parenteral therapy is usually recommended as well.
Other neurotropic viruses
- Several other viruses have a tropism for neural cells.
- CMV is the most common acquired cause of sensorineural hearing loss and less commonly, can cause meningoencephalitis.
- In CMV meningoencephalitis, abnormalities range from neuronal migration anomalies form teratogenicity, to periventricular white matter abnormalities.
- Toxoplasmosis is an inflammatory parasitic infection that principally destroys the CNS and the eye in the newborn.
- The classical manifestations of congenital toxoplasmosis includes: (1) chorioretinitis, (2) meningoencephalitis, (3) diffuse intracranial calcification, and (4) hydrocephalus.
- Enteroviral and parechoviral meningoencephalitis are typically acquired intrapartum and mostly affect the white matter and look like PVL on MRI.
- Enterovirus and parechovirus.
- Zika is a mosquito born virus that in 2014 suddenly resulted in cases of congenital microcephaly in Brazil and other countries.
- Clinically, the striking features are profound congenital microcephaly with overlapping sutures and redundant scalp tissue.
Neonatal Stroke
Neonatal stroke can be defined as a cerebrovascular injury that occurs around birth. It can be focal or multifocal and may include both ischaemic and haemorrhagic injury. Neonatal stroke is frequently referred to as perinatal cerebral injury of ischaemic origin.
Delayed seizure onset and clinically observed focal seizures are predictors of stroke in neonates with seizures.
Two common subtypes are identified, perinatal arterial ischaemic stroke (PAIS) and cerebral sinovenous thrombosis (CSVT). There is still discussion about which other patterns of injury should be regarded as perinatal stroke. Periventricular haemorrhagic infarction (PVHI), which is more commonly seen in preterm infants, is also regarded as being part of the stroke spectrum.
Diagnosis
An amplitude integrated EEG, (aEEG) should be obtained to evaluate seizures.
Full channel EEG is helpful to localise the origin of seizures.
Laboratory studies, to rule out other pathology, including hypoglycemia, electrolyte disturbance, infection and metabolic syndromes.
Neuroimaging
Cranial ultrasound is a useful non-invasive test, however it may not accurately diagnose stroke as it may appear normal shortly after symptom onset.
MRI (magnetic resonance imaging) is the most sensitive modality for detecting abnormalities of signal intensity.
Neonatal neuroimaging plays an important role in both diagnosis and prognosis and may be useful in predicting motor outcome.
Risk factors
- It is thought that the presence of a patent foramen ovale in the perinatal period allows passage of thrombi from the placenta or venous circulation into the cerebral circulation, resulting in arterial occlusion.
- Male infants are more often affected and PAIS more often involves the left hemisphere.
- Other risk factors include primigravid mothers, chorioamnionitis, complicated deliveries, intrauterine growth restriction.
- Congenital heart disease.
- Prothrombotic factors may play a role, although recurrent of ischemic stroke is rare, suggesting a multifactorial aetiology.
Management
Supportive care
Continuous EEG monitoring and treatment of seizures
Anticoagulants may be useful to prevent propagation of thrombus in cases of CSVT without evidence of cerebral haemorrhage.
Prognosis
Both short and long-term outcomes mainly depend on the extent and location of the stroke.
In PAIS, unilateral spastic cerebral palsy is the most frequent sequela and has been reported in up to 50% of cases, depending on stroke location.
Cognitive outcome is more variable. Seizures may also recur.
There are fewer studies on outcome following CSVT and reported outcomes vary.
Long-term outcome studies suggest that 60-80% of all infants develop some kind of deficit. Both motor and cognitive abnormal outcomes have been reported.
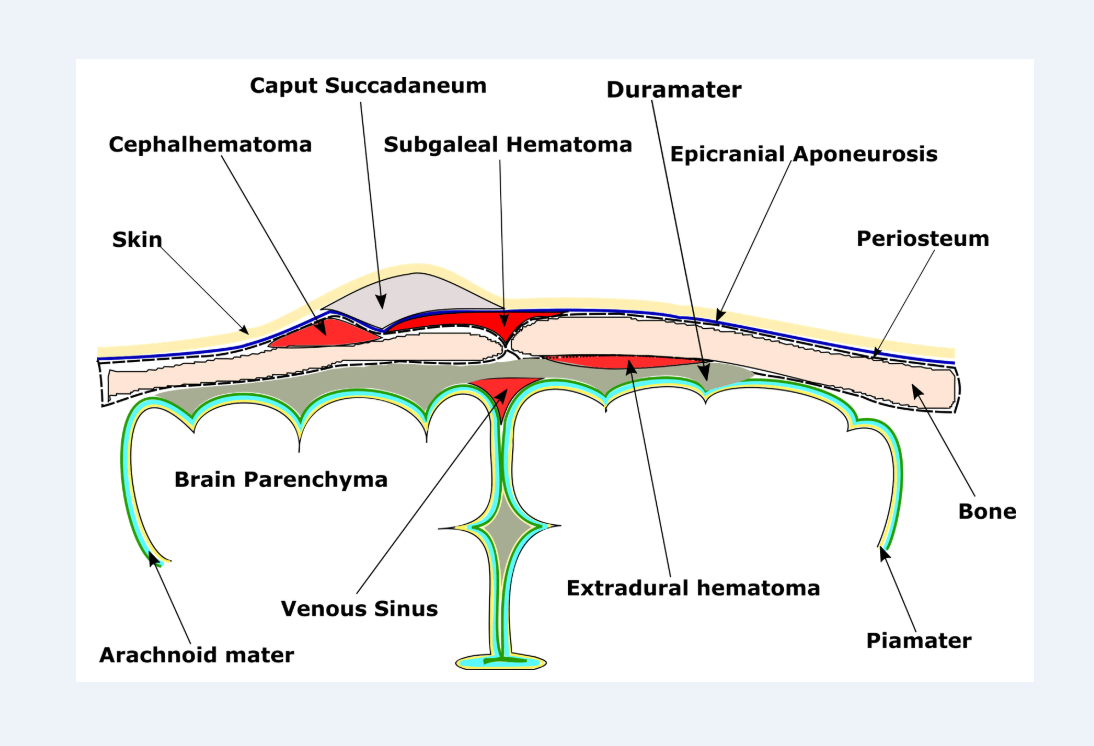
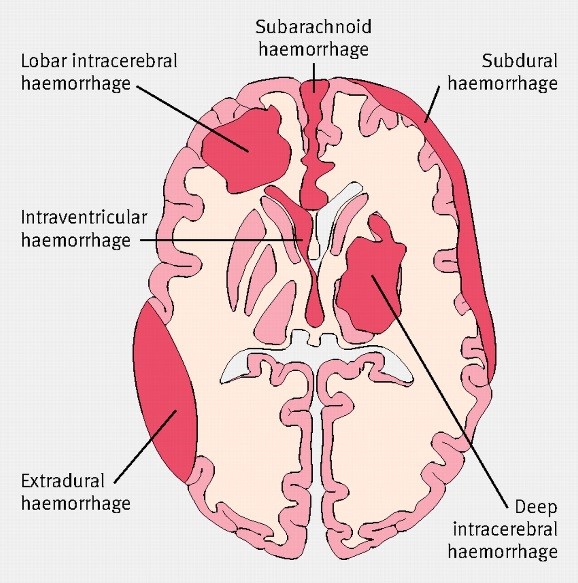
Intracranial Hemorrhage in Term and Near-Term Infants
Risk factors/causes include primiparity, difficult and instrumental deliveries, intrapartum asphyxia, major surgery, bleeding disorders, and intracranial aneurysms.
Symptoms are similar to those of postasphyxial HIE but tend to be milder (eg, Apgar scores higher and less need for resuscitation) or start later (eg, seizure onset on day 2 or 3 rather than day 1); in addition, coexisting asphyxia is often present.
- Coexisting trauma of scalp, face and skeleton, and adrenal hemorrhage is common.
- US followed by MRI scan are the preferred diagnostic modes.
- Management, except of massive bleeds, is conservative. The results of surgery of posterior fossa bleeds have been disappointing.
Brachial plexus palsy
Shoulder dystocia and breech delivery, especially of large for gestational age infants, are the most common causes of brachial plexus palsy in new- born infants. The incidence varies from 1.5–4/1,000 deliveries. The injury takes the form of neuropraxia, axonotmesis, neurotmesis, or avulsion of the root.
The most common site is the upper part of brachial plexus resulting in classic Erb’s palsy, a characteristic external rotation at the elbow, with absent movements at the wrist and a pronated hand. The clinical presentation during the neonatal period is similar irrespective of the degree/severity of injury and its prognosis. Fractured clavicle or humerus should be ruled out as the cause of poor limb motility, or as coincident lesions.
A good functional recovery is reported in approximately 80 to 90%; 10 to 20% of patients have residual deficits. Studies describing the natural history are lacking. Long- term consequences include loss of limb function, orthopedic abnormalities, cosmetic deformities, and psychosocial effects.
Surgical repair in the form of resection of neuroma and nerve grafting has been advocated for infants who have not recovered biceps function by 3 months. Surgical reconstruction has been reported to produce better results than non-intervention, using historical controls with similar severity of lesions. No randomized controlled studies have been performed yet.
A multidisciplinary approach involving neurosurgeon, orthopedic surgeon, physiotherapist, occupational therapist, and community pediatrician is warranted for infants who do not show demonstrable signs of recovery by 6 to 8 weeks of age.
Neonatal Hypotonia:
- Muscle tone is the continuous and passive partial contraction of the muscles or the resistance to passive movement around a joint. Hypotonia results in impaired ability to sustain postural control against gravity, with increased range of movement of joints.
- Assessment of muscle tone should be performed while infant is positioned at midline in an awake and resting state.
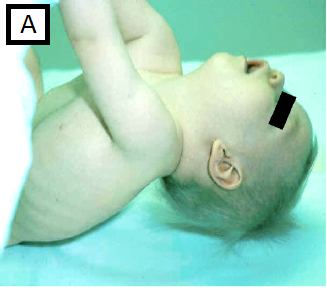
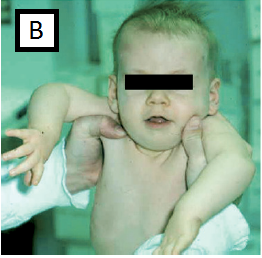
Common clinical signs:
- Significant head lag on traction or pull-to-sit maneuver, fig A
- Vertical suspension test e feeling of ‘slipping through the hands’ when the infant is held under the arms, fig B
- In supine, frog-leg posture (legs fully abducted and arms lying beside the body), fig C
- Rag-doll posture on ventral suspension, fig D
- Scarf sign (elbow cross midline), fig E
- Other associated clinical findings: flat occiput, congenital dislocation of the hips, arthrogryposis, or paradoxical breathing.
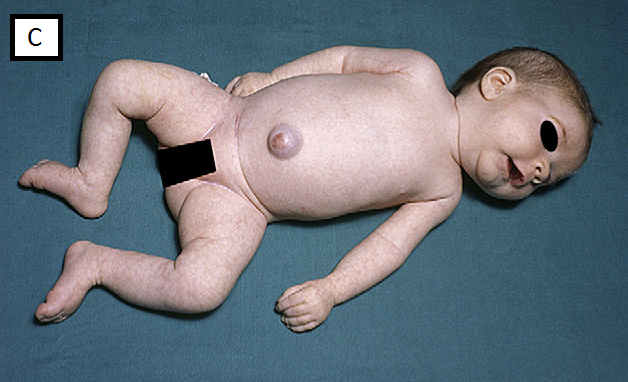
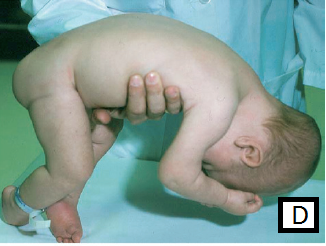
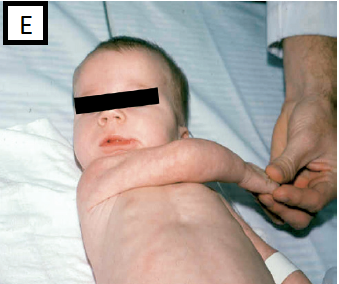
A. Clinical features suggestive of hypotonia of central origin:
- The state of alertness: decreased level of consciousness and activity.
- Microcephaly or macrocephaly.
- Dysmorphic features or organ malformations implying a syndrome.
- Normal or brisk tendon reflexes (axial weakness is commonly significant, with preservation of limb power and hyper-reflexia).
- Fisting of hands.
- Features of pseudobulbar palsy and other cranial nerve affection (eg failure to elicit facial expressions or track visually).
- Crossed adductor response or scissoring on vertical suspension.
B. Clinical features suggestive of hypotonia peripheral origin:
- Reduced fetal movements and polyhydramnios.
- Family history of neuromuscular disorders/maternal myotonia.
- Reduced or absent spontaneous antigravity movements.
- Reduced or absent deep tendon jerks and increased range of joint mobility
- Myopathic facies (open mouth with tented upper lip, poor lip seal when sucking, lack of facial expression, ptosis and restricted ocular movements).
- Muscle or tongue fasciculation (Anterior horn cell lesion eg, spinal muscular atrophy).
- Poor swallowing ability causing drooling and oropharyngeal pooling of secretions.
- Weak cry and abnormal breathing pattern.
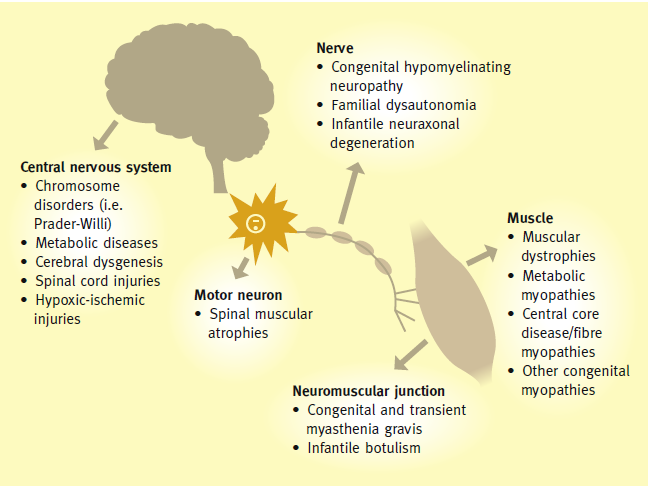
- Central causes of hypotonia are more common than peripheral (60-80% vs 15-30%) and there is evidence that 50% of diagnoses can be made by history and examination alone.
- Depending upon various clues, genetic tests could be done as a first line investigation.
- Recently, array CGH (Comparative Genomic Hybridization) has come up as a powerful diagnostic tool having detection rate of 5-17% in cases of normal karyotype.
- Whole Exon Sequencing (WES) is promising in detecting causal genetic variants of rare monogenic disorders.
Diagnostic clues for central causes of hypotonia:
|
Condition |
Clinical Features |
Diagnostic Investigation |
| Hypoxic Ischemic Encephalopathy | – Delivery complications, evidence of asphyxia and low Apgar scores | – Brain MRI
– aEEG/EEG |
| Cerebral malformations
(eg, holoprosencephaly, lissencephaly, agyria, pachygyria) |
– Seizures, microcephaly | – Brain MRI |
| CNS infection | – History suggestive of congenital or acquired infection, HSM, thrombocytopenia | – TORCH screen, eye exam, CSF analysis, brain MRI/CT |
| Chromosomal disorders:
– Prader-Willi syndrome (PWS) – Williams syndrome |
– Dolicocephaly, almond shaped eyes, undescended testes, feeding difficulties
– Epicanthic folds with periorbital fullness, stellate irises and midface hypoplasia |
– FISH testing for deletion on chromosome 15q13
and if negative by MLPA (Multiplex Ligation-dependent Probe Amplification) testing – DNA-based methylation testing within the PWS critical region will detect more than 99% of individuals with PWS, which arises from the loss of function of paternally expressed 15q11eq13 genes – Fluorescent in situ hybridization (FISH), detects the elastin deletion on chromosome 7 in more than 98% of individuals with Williams syndrome |
| – Trisomy 21 | – Clinical features of trisomy 21 | – Karyotype |
| Peroxisomal disorders:
– Zellweger syndrome – Neonatal adrenoleukodystrophy |
– Renal cysts, high forehead, wide fontanelles
– Hepatomegaly, retinitis pigmentosa ( neonatal adrenoleukodystrophy) |
– Very long chain fatty acids (VLCFA) |
| Inborn error of metabolism | – Family history
– Abnormal odor – Multisystem affection – Hypoglycemia |
– Raised ammonia: urea cycle defects, organic acidemias or fatty acid oxidation dis.
– Amino acids in blood: aminoacidopathies – Urine organic acids: organic acidemias – Acylcarnitine in the plasma: fatty acid oxidation defects – Low serum Uric acid and high sulfite and Sulfocysteine urine in molybdenum cofactor deficiency |
Diagnostic clues for peripheral causes of hypotonia:
|
Condition |
Clinical Features |
Diagnostic Investigation |
| Spinal cord lesion:
– Trauma – Meningomyelocele |
– History and physical examination | – Brain and spine US/MRI |
| Anterior horn lesion:
Spinal muscular atrophy (commonest peripheral cause of hypotonia) o SMA type 1(commonest ): Werdnig- Hoffmann (AD) o Chronic SMA: Type 2 & 3 o SMARD 1 (AR) |
-Generalized weakness, affecting the legs more than the arms and
proximal more than distal, with sparing of the diaphragm (abdominal breathing is evident with marked intercostal muscle weakness) and facial muscles -Tongue fasciculations -Absent DTR |
– Normal creatinine kinase
– Denervation on EMG – Confirmed (95-98% of cases) by testing for the homozygous deletion of exon 7 in the telomeric survival motor neurone (SMN1)gene |
| Neuromuscular junction:
Transient myasthenia gravis (MG) |
– 10 and 20 % of infants born to women with MG develop neonatal MG, caused by the maternal IgG antibodies to AChR crossing the placenta (80% present at first 24 h)
– Arthrogryposis, feeding difficulties, recurrent apnoeic/ choking episodes, ophthalmoplegia, ptosis, facial weakness, weak cry – Fatiguability, ptosis: both may be present in mother – In transient form: after the abnormal antibodies disappear from the blood and muscle tissue, the infants regain normal strength (2- 4 months) and are not at increased risk of developing MG in later – Congenital form is non-immune, rare AR present in 1st year of life |
– EMG: repetitive nerve stimulation shows
an electro-decrement of more than 10% in amplitude of the compound muscle action potential – Serum antibodies in mother and neonate – Administration of acetylcholinesterase agents transiently correct the neuromuscular transmission defect |
| Muscles:
Congenital myotonic dystrophy |
-There is no myotonia in the infant, but examination of the mother may reveal grip or percussion myotonia and facial weakness
– Polyhydramnios, preterm birth hypotonia, talipes, facial weakness with a tented upper lip and high arched palate. The cry and suck are weak, need prolonged respiratory support – Hypotonia and muscle strength improve with time, but facial weakness persists. Cognitive delay is significant -MRI brain might show punctate white matter injury |
– Genetic test: AD, caused by maternally transmitted expanded CTG trinucleotide repeat (more than 200) in the DMPK gene
– Severity of symptoms correlates with number of repeats |
| Congenital myopathy:
a) Myotubular or centronuclear myopathy b) Nemaline myopathy c) Central core myopathies |
a) Myotubular or centronuclear:
The diagnosis should be considered in a male with neonatal hypotonia and weakness, particularly if family history suggests X-linked inheritance and/or length or head circumference are more than 90th centile with long fingers and toes b) Nemaline: congenital hypotonia, generalized weakness with respiratory involvement and swallowing difficulties c) Central core: congenital hypotonia, hip dislocation and proximal weakness with delayed motor milestones and susceptibility to malignant hyperthermia |
a) Histology shows small rounded fibers with centrally located nuclei resembling fetal myotubes . Molecular testing of MTM1 detects mutations in up to 98%.
b) Characterized by nemaline rods seen on histology. The most common genes are nebulin and ACTA1 c) Characterized on muscle biopsy by specific rounded areas lacking oxidative enzyme activity in type one fibers. Over 90% of cases are caused by mutations in the RYR1 gene, inherited as an AD NB. Traditionally a specific diagnosis is based on the structural features of the muscle biopsy, but these conditions are clinically and genetically heterogeneous: multiple genes can result in the same muscle pathology and clinical phenotype |
| – Congenital muscular dystrophy | – Hypotonia and joint contractures
-T2-weighted MRI shows increased white matter signal intensity |
– Muscle biopsy demonstrates a dystrophic process (signs of degeneration and regeneration)
– Markedly elevated CK (more than 1000 iu/l) – Gene testing: mainly due to AR genes of the extracellular matrix. The commonest condition is MDC1A, with primary deficiency of laminin-alpha 2 (merosin negative) |
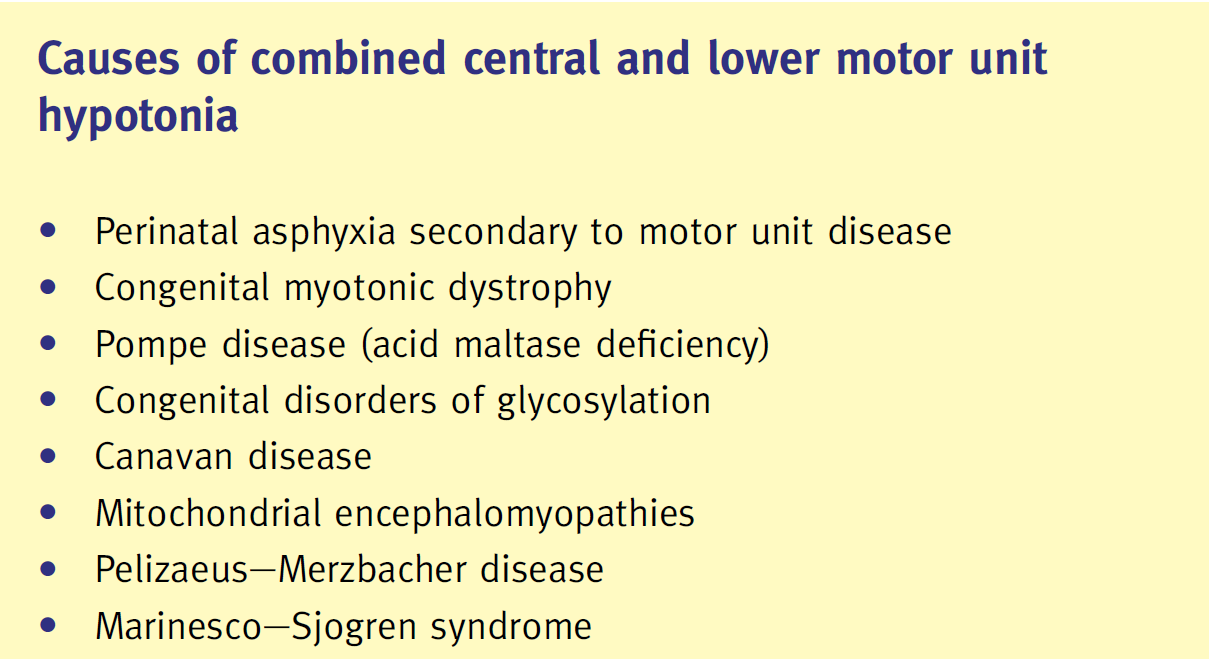
Diagnostic clues for causes of combined central and peripheral hypotonia:
|
Condition
|
Clinical Features |
Diagnostic Investigation
|
| Pompe disease:
(deficiency in the lysosomal enzyme acid alpha glucosidase (GAA), resulting in abnormal glycogen deposition in heart, brain and muscles) |
– Hypertrophic cardiomyopathy, hypotonia, weakness and respiratory insufficiency | – Deficient GAA activity in blood, fibroblasts or muscle biopsy |
| Congenital disorders of glycosylation CDG:
(AR multi-system diseases caused by defects in protein glycosylation) |
– Central hypotonia and weakness, infants with CDG type 1a have dysmorphic features, inverted nipples and abnormal fat distribution | – Isoimmune electrophoresis for transferrin (abnormal pattern in CDG) |
| Mitochondrial disorders:
(caused by dysfunction of the respiratory chain, which is predominantly responsible for oxidative phosphorylation and the production of ATP) |
– Hypotonia with encephalopathy, cardiomyopathy
and external ophthalmoplegia – Raised lactate in blood/CSF |
– Genetic testing sequencing the whole mitochondrial genome or mitochondrial DNA point-mutation screening is now available
– Muscle biopsy can be extremely valuable with ragged red fibers, accumulation of structurally abnormal mitochondria, and cytochrome-c-oxidase negative fibers |
Advances in therapy:
- Use of acetylcholinesterase inhibitors, 3,4-diaminopyridine and Ephedrine, Salbutamol or Fluoxetine in some congenital myasthenic syndromes.
- Antisense oligonucleotide therapy has been under trial for Duchenne muscular dystrophy.
- Enzyme replacement therapy (myozyme) for infantile Pompe disease.
- Trials of treatment for SMA are underway.

Further reading
Neurology of the Newborn, 6th Edition
Van der Aa NE, Benders MJNL, Groenendaal F, de Vries Ls. Neonatal stroke; a review of the current evidence on epidemiology, pathogenesis, diagnostics and therapeutic options. Acta Paediatrica 2014;103:356-364.
