Neonatal Jaundice
Andrew James BSc, MBChB, MBI, FRACP, FRCP
The material presented here was first published in the Residents’ Handbook of Neonatology, 3rd edition, and is reproduced here with permission from PMPH USA, Ltd. of New Haven, Connecticut and Cary, North Carolina.
Neonatal jaundice, which usually presents as an unconjugated hyperbilirubinaemia, is one of the most common physical signs observed amongst newborn infants. Approximately 60% of term newborn infants and 80% of preterm infants will have visible jaundice during the first week of life. The jaundice, which is almost universal, has been called physiological jaundice. It is a transient physiological phenomenon for most newborns that may be regarded as a manifestation of their ongoing adaptation to the extrauterine environment.
Jaundice may be a sign of pathology, and demands appropriate evaluation and rational management. It is important to appreciate that a jaundiced newborn’s symptoms may be attributed to the jaundice when in fact they are due to other pathology. The evidence for managing jaundice is poor especially in unhealthy newborns and preterm infants. The full spectrum of adverse outcomes for hyperbilirubinaemia and the safety of many interventions are not known.
The clinical challenge
Despite improvements in neonatal care and the virtual absence of classic kernicterus, safe serum bilirubin levels have not been established with absolute certainty. The data from numerous studies of bilirubin toxicity are so complex that it has been difficult to derive a rational approach for the management of the jaundiced neonate. Numerous guidelines for the management of jaundiced neonates have been published but their effectiveness has not been validated by properly designed clinical trials [American Academy of Pediatrics Subcommittee on Hyperbilirubinaemia, 2004; Canadian Pediatric Society Fetus and Newborn Committee, 2007].
Acute bilirubin encephalopathy and kernicterus, although less common than previously, are still occurring [Sgro et al, 2012]. The key root causes that appear to be significant contributors to the reported cases of kernicterus include:
- failure to recognise important risk factors including gestational age < 38 weeks and breast feeding
- failure to evaluate jaundice in the first 24 hours of life
- underestimation of the severity of the jaundice by visual assessment
- failure to respond to parental concerns about jaundice, poor feeding and changes in newborn behaviour
- failure to provide timely follow-up
Extreme hyperbilirubinaemia is not rare: in 2002-04 approximately 1 in 2000 Canadian infants had peak serum bilirubin concentrations greater than 425 micromol/L [Sgro et al, 2006].
Bilirubin metabolism
Several biophysical processes are involved in the production, transport, metabolism and clearance of bilirubin. These include the following:
- degradation of red blood cells
- binding of bilirubin to albumin
- transport of bilirubin to the liver
- uptake by the liver
- conjugation within the liver
- hepatic excretion and transfer into bile
- intestinal transport
All the biophysical processes involved in the synthesis, distribution, metabolism and excretion of bilirubin are illustrated in Figure 1.

Figure 1. Synthesis, distribution, metabolism and excretion of bilirubin
The abnormal functioning of any of these biophysical processes may cause neonatal jaundice that progresses to severe hyperbilirubinaemia, acute bilirubin encephalopathy and kernicterus. There are several genetically determined enzyme deficiencies that interfere with bilirubin synthesis, metabolism and excretion [Kaplan M, Hammerman C, Maisels MJ., 2003]. Glucose-6-dehydrogenase deficiency (G6PD) is the commonest of these conditions.
Bilirubin neurotoxicity
Bilirubin induced brain injury occurs with a spectrum of clinical features from reversible neurophysiological and behavioural changes through to permanent structural changes.
Possible mechanisms that facilitate bilirubin entry into the brain and binding to neuronal cell membranes include acidosis, reduced binding of bilirubin to serum albumin, and disruption of the blood-brain barrier [Table I].

Table 1. Mechanisms of that facilitate bilirubin entry into the brain
The following drugs, which compete with bilirubin for binding sites on albumin, should be avoided in newborns with significant jaundice.
-
- Analgesics including salicylates and ibuprofen
- Antimicrobials, including cephalosporin and sulfonamides, but not trimethoprim/sulfamethoxazole (co-trimoxazole)
- Radiographic contrast media
Bilirubin causes general cellular injury by inhibiting mitochondrial enzymes, interfering with DNA synthesis, inducing DNA-strand breakage, and inhibiting protein synthesis and phosphorylation [Chuniaud et al, 1996]. A model that describes the pathophysiology for bilirubin neurotoxicity is presented in Table 2.

Table 2. A model for bilirubin neurotoxicity
Symptoms and signs may be subtle and non-specific. The clinical features of bilirubin encephalopathy vary depending on the age of the infant and the degree of hyperbilirubinaemia. Three terms are commonly used to describe bilirubin neurotoxicity.
- Acute bilirubin encephalopathy
Acute signs of bilirubin encephalopathy include lethargy, poor feeding, temperature instability, and hypotonia, leading to arching of the head, neck and back [opisthotonos], spasticity and seizures. Death may follow. Many of the survivors will have permanent brain injury.
The brain MRI shows high signal of the globus pallidus and MR spectroscopy has a characteristic metabolic signature [Oakden et al, 2005]. These changes may be transient and therefore, should not be interpreted as definite evidence of permanent brain injury.
- Chronic bilirubin encephalopathy/Clinical kernicterus
The classical signs of kernicterus constitute a tetrad consisting of extrapyramidal disturbances, auditory abnormalities, gaze palsies and dental enamel dysplasia [Perlman, 1960]. There may also be impairment of neurocognitive functioning.
- Pathological kernicterus
This term refers to the findings at autopsy in babies dying with the acute disease. It is characterised by bilirubin deposition, causing yellow discoloration, and neuronal death in the basal ganglia, cerebellum and deep nuclear structures, including subthalamic structures, the hippocampus and brainstem. The findings in subjects with chronic bilirubin encephalopathy show chronic brain pathology in the same sites without yellow staining.
Causes of jaundice:
Common causes of neonatal jaundice during the first week of life include those physiological mechanisms that are unique to the newborn infant, immaturity of hepatic enzymes, and breast feeding.
Physiological jaundice is first observed in the face when the serum bilirubin (SBR) is at least 80-120 micromol/L. There is a slow cephalocaudal progression — from face to trunk to the limbs, which are usually spared. The rate of rise of bilirubin is less than 8.5 micromol/L/h. The measurement of serum bilirubin levels is necessary to determine the severity of the jaundice and establish an appropriate plan for management, simply because, the visual assessment of the severity of jaundice by the human eye is often inaccurate.
The biological mechanisms of physiological jaundice as related to the processes of bilirubin synthesis, transfer, hepatic conjugation and excretion are listed in Table 3.

Table 3. Mechanisms of physiological jaundice
SBR levels greater than the 95th percentile for postnatal age, which are shown in Figure 2, should not be regarded as physiological without further investigation and exclusion of pathological causes. Most mature newborn infants are discharged from hospital before physiological jaundice reaches its peak at 3-5 days.

Figure 2. Nomogram for designation of risk for hyperbilirubinaemia in healthy newborns (GA > 36 weeks and birthweight > 2,000 g, or GA > 35 and birthweight > 2,500 g) based on the hour-specific serum bilirubin values. The serum bilirubin was measured before discharge, and the zone in which the value fell predicted the likelihood of a subsequent bilirubin value exceeding the 95th percentile. [Bhutani et al, 1999; American Academy of Pediatrics Subcommittee on Hyperbilirubinemia, 2004].
Although most jaundice is mild and physiological in origin, it cannot be safely assumed to be either. Atypical jaundice — early onset jaundice, a rapidly rising bilirubin, prolonged jaundice, late onset jaundice or conjugated hyperbilirubinaemia — is likely to reflect pathology. Physiological and pathological jaundice overlap and furthermore, both may be occurring simultaneously.
Jaundice occurring within the first 24 hours of life must be considered to be pathological until proven otherwise.
Conditions that cause early onset neonatal jaundice include:
- increased degradation of red blood cells as a direct consequence of either enzymatic or structural defects of the red blood cell
- abnormality of hepatic membrane receptors and/or hepatic enzymes
- impairment of hepatobiliary excretion
- increased enterohepatic circulation of bilirubin
- breast feeding jaundice
Conditions that cause prolonged jaundice, late onset jaundice, or conjugated hyperbilirubinaemia include:
- breast milk jaundice
- congenital hypothyroidism
- congenital infection
- metabolic disorders
- structural abnormalities of the liver and hepatobiliary tract
- Clinical assessment
The evaluation of the jaundiced newborn must include a thorough history and physical examination, measurement of the transcutaneous and/or serum bilirubin (SBR), and other investigations as indicated. The possibility of an acute haemolytic process must always be considered, especially if there is visible jaundice within the first 24 hours of life.
Jaundice can be appreciated once the SBR is greater than 75-80 micromol/L. The jaundiced newborn is easily recognised if physicians and nurses are observant. A clinical assessment for jaundice should be performed on all newborn infants whenever the infant’s vital signs are assessed but no less than every 8-12 hours.
Clinical examination cannot accurately predict the SBR: the visual assessment of the severity of jaundice by the human eye is often inaccurate. Any doubt about the level of jaundice should be resolved by transcutaneous and/or serum measurements.
Transcutaneous estimation of SBR may be used for screening. The estimate is influenced by race and postnatal age. If it is > 200 micromol/L, the serum bilirubin should be measured. Normal values for transcutaneous measurements are shown in Figure 3. There is a tendency for transcutaneous measurements to underestimate the SBR whenever the SBR is high.
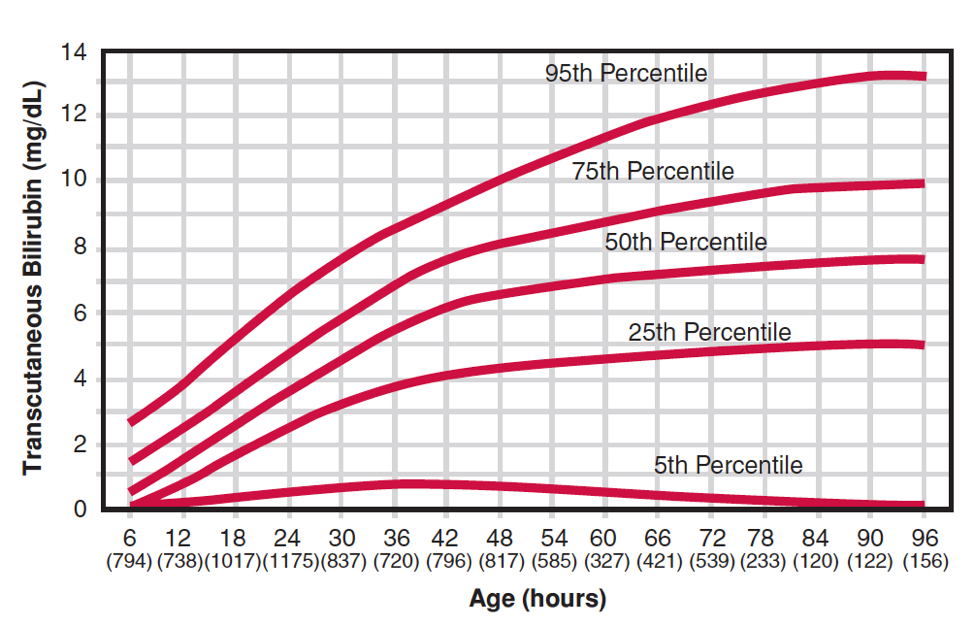
Figure 3. Transcutaneous bilirubin levels of healthy white infants > 35 weeks during the first 96 hours of life [Maisels and Kring, 2006].
All NICUs should have established protocols for the visual assessment of jaundice. These protocols should include the circumstances in which nursing staff can obtain either a transcutaneous, if available, and/or SBR measurements without a physician’s order.
Laboratory investigations
The following laboratory investigations are recommended for the assessment of the jaundiced newborn infant.
- total, unconjugated and conjugated bilirubin concentrations [conjugated SBR concentrations < 20% total SBR concentrations are considered normal by current consensus-based guidelines] [American Academy of Pediatrics Subcommittee on Hyperbilirubinemia, 2004]
- maternal blood groups and red blood cell antibody screen
- infant blood group and direct antibody test (DAT or Coombs test)
- haemoglobin, rwhite blood cell count and differential, platelet and reticulocyte counts
- blood film for evidence of haemolysis and assessment of red blood cell morphology
Additional laboratory investigations and organ imaging are recommended for the assessment of late onset jaundice, prolonged jaundice and conjugated hyperbilirubinaemia.
- investigation for sepsis including urinary tract infection
- investigation for congenital infections
- investigation for G6PD deficiency, galactosaemia and congenital hypothyroidism
- investigation for rare metabolic disorders
- investigation of the structure and function of the liver and biliary tract [abdominal ultrasound, HIDA scan]
Management
The need for active treatment of the jaundiced newborn is determined by the estimated risk for bilirubin neurotoxicity as inferred from the postnatal age specific SBR and/or the rate of rise of the SBR, and other biophysical or pathophysiological factors.
Interpret all SBR levels according to the infant’s postnatal age in hours. Use the total SBR for clinical decision making.
Infants < 38 weeks gestational age should not be managed as if they were mature, full-term infants. These infants, particularly those who are breast fed, are at much higher risk of developing severe hyperbilirubinaemia and require much closer supervision and monitoring.
The traditional, well established modalities of treatment for neonatal jaundice are phototherapy and exchange transfusion.
Phototherapy, which is the primary treatment for neonatal jaundice, has proven to be a generally safe procedure although rare complications can occur. The purpose of phototherapy is to prevent the need for exchange transfusion.
Exchange transfusion thresholds are positioned to keep the SBR below levels at which kernicterus has been reported. In most situations, exchange transfusion is recommended only after phototherapy has failed to prevent the SBR rising to exchange thresholds.
Phototherapy
The purpose of phototherapy is to prevent the need for exchange transfusion. Phototherapy should be commenced 75-100 micromol/L below the recommended level for exchange transfusion. With intensive phototherapy the total SBR should decline by 17-34 micromol/L within 4-6 hours.
Phototherapy induces the photo-oxidation of unconjugated bilirubin in the skin to water soluble photoisomers that are excreted in the bile and urine without conjugation.
Optimal phototherapy demands the use of high levels of irradiance in the 425-475 nm band delivered to as much of the infant’s surface area as possible from a distance of
15-20 cm from the infant [Ip et al, 2002]. Intensive phototherapy is twice as effective as standard phototherapy.
Phototherapy has proven to be a generally safe procedure. Potential complications include temperature instability; mild fluid loss as a consequence of transepidermal losses and increased stools; skin rash and tanning of the skin; retinal damage and burns. The only contraindications to the use of phototherapy are conjugated hyperbilirubinaemia, congenital erythropoietic porphyria or a family history of that condition, and concurrent medication known to induce photosensitivity.
Hydration is a treatment only for jaundice attributed to dehydration. It has been shown to be effective in the treatment of dehydrated near-term and term infant presenting with jaundice at several days of age [Mehta et al, 2005].
Infants with cholestatic jaundice exposed to phototherapy may develop the bronze infant syndrome — dark, grey-brown discoloration of the skin, serum and urine.
The SBR levels at which phototherapy is recommended for mature or near mature newborn infants (GA ≥ 35 weeks) are documented in Table 4 and Figure 4.

Table 4. Recommended SBR levels for commencing phototherapy for infants GA ≥ 35 weeks

Figure 4. Recommended SBR levels for commencing phototherapy for infants GA ≥ 35 weeks
The SBR levels at which phototherapy is recommended for immature newborn infants are documented in Table 5.

Table 5. Recommended SBR levels for commencing phototherapy for immature newborn infants
There is no established standard for discontinuing phototherapy. The SBR level at which phototherapy is discontinued should be influenced by its distance from the threshold for exchange transfusion, the cause of the hyperbilirubinaemia, the response to phototherapy and the age at which the phototherapy was commenced. The SBR, which is usually lower than the level at which phototherapy was commenced, often increases after phototherapy is discontinued. This phenomenon is known as “rebound” jaundice and usually occurs within 12 hours of discontinuing phototherapy. Further phototherapy may be indicated.
Exchange transfusion
The SBR threshold for exchange transfusion should be individualised and must consider the factors that alter the potential for the development of bilirubin toxicity. These include the presence of metabolic acidosis, hypoalbuminaemia, active haemolytic disease, and clinical neurological signs consistent with acute bilirubin encephalopathy.
The SBR levels at which exchange transfusion is recommended for mature or near mature newborn infants [GA ≥ 35 weeks] are documented in Table 6 and illustrated as Figure 5.

Table 6. Recommended exchange transfusion levels for infants GA ≥ 35 weeks

Figure 5. Recommended exchange transfusion levels for infants GA ≥ 35 weeks
The SBR levels at which exchange transfusion is recommended for immature newborn infants are documented in Table 7.

Table 7. Recommended SBR levels for commencing phototherapy for immature newborn infants
Important additional criteria for commencing an exchange transfusion, especially for those with suspected and or proven haemolytic disease, include the following:
- cord bilirubin > 85 micromol/L
- cord hemoglobin < 120 g/L
- rapidly rising bilirubin: rate of bilirubin increase > 17 micromol/L/h
Exchange transfusion is often performed using the “push-pull” technique through a single umbilical venous catheter traversing the ductus arteriosus with the tip in the inferior vena cava. A two-vessel procedure using an umbilical arterial catheter for withdrawal of blood and an umbilical venous catheter for transfusion of blood facilitates a continuous, isovolumetric method of exchange transfusion [Martin, 1973].
Significant morbidity occurs in as many as 5% of exchange transfusions. Complications include an imbalance between blood volume transfused and blood withdrawn as a consequence of errors in technique and/or hypotension with large-volume blood withdrawal aliquots in a single catheter exchange; hypocalcaemia, thrombocytopenia and metabolic acidosis; necrotising enterocolitis; air and thromboembolism causing myocardial infarction or stroke; and the risks of transfusion related infection. Exchange transfusion related mortality has been reported as approximately 3 per 1000 procedures [Kennan et al, 1995].
Exchange transfusions should be performed in a level III neonatal intensive care unit (NICU), supervised by a neonatologist with knowledge of the complications of exchange transfusion and experience with the procedure. This may require pre-emptive transfer of a jaundiced neonate to the appropriate level III NICU before exchange transfusion levels have been reached. The urgency for starting an exchange transfusion depends not only on the SBR level, but also the presence and severity of neurological signs. Confirmation of the SBR level is desirable before starting the exchange, but may be forgone in emergencies — infants with definite signs of neurotoxicity. In emergencies, if the most appropriate blood is unavailable, consider the use of group O Rhesus-negative blood.
Innovative therapies
Intravenous gammaglobulin
Intravenous immunoglobulin (IVIG) acts as a competitive inhibitor for antibodies that cause haemolysis. The use of IVIG in newborns with isoimmune haemolytic jaundice reduces the need for exchange transfusion [Alcock, Liley 2002; Gottstein, Cooke 2003].
Efficacy has been shown for newborn infants with Rhesus and other isoimmune haemolytic jaundice with the following:
- High cord bilirubin and/or low haemoglobin
- Rapid postnatal rise in SBR despite phototherapy
- Approaching threshold for exchange transfusion
The anticipated benefit must be balanced against the risk of using blood products from multiple/pooled donors.
The recommended dose is 0.5-1.0 g/kg infused over 2 hours. A second dose may be administered after 12 hours.
Metalloporphyrin
The inhibition of haeme oxygenase and bilirubin production by Sn-Mesoporphyrin represents a novel pharmacological intervention for the prevention and treatment of neonatal jaundice [Martinez et al, 1999]. Haeme oxygenase inhibitors suppress the production of bilirubin as shown in Figure 1. Preliminary evidence indicates efficacy but safety data are lacking. The role of this newly developed therapy in the management of neonatal jaundice has not been clearly established yet [Dennery PA, Seidman DS, Stevenson DK., 2001; Dennery PA, 2002].
Key recommendations
- A clinical assessment for jaundice should be performed on all newborn infants whenever the infant’s vital signs are assessed but no less than every 8-12 hours.
- All NICUs should have established protocols for the visual assessment of jaundice. These protocols should include the circumstances in which nursing staff can obtain either transcutaneous and/or blood SBR measurements without a physician’s order.
- If there is any doubt about the level of jaundice, the blood SBR should be measured.
- Measure the blood SBR on every baby who is jaundiced in the first 24 hours of life.
- Interpret all SBR levels according to the infant’s postnatal age in hours.
- Infants < 38 weeks gestational age should not be managed as if they were mature, full-term infants. These infants, particularly those who are breast fed, are at much higher risk of developing severe hyperbilirubinaemia and require much closer supervision and monitoring.
- Follow recognised guidelines for the use of phototherapy and exchange transfusion.
- The purpose of phototherapy is to prevent the need for exchange transfusion.
- Any infant whose SBR is greater than 425 micromol/L should be treated immediately.
- Perform a systematic assessment of all infants, prior to discharge, for the subsequent risk of hyperbilirubinaemia. If appropriate follow-up cannot be assured, assess the risk for severe hyperbilirubinaemia, and obtain a blood SBR.
References
- Kirpalani, H., Moore, A.M. and Perlman, M., 2007. Residents handbook of neonatology. PMPH-USA
- Alcock GS, Liley H. Immunoglobulin infusion for isoimmune haemolytic jaundiced neonates. Cochrane Database Sys Rev 2002;(3):CD003313.
- American Academy of Pediatrics Subcommittee on Hyperbilirubinaemia. Management of hyperbilirubinaemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114:297-316.
- Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics 1999;103:6-14.
- Canadian Pediatric Society Fetus and Newborn Committee. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks’ gestation). Pediatr Child Health 2007;12 (Suppl B):1B-11B. (Reaffirmed February 28, 2018).
- Available at https://www.cps.ca/en/documents/position/hyperbilirubinemia-newborn Last accessed May 25, 2019.
- Chuniaud L, Dessante M, Chantoux F, Blondeau JP, Francon J, Trivin F. Cytotoxicity of bilirubin for human fibroblasts and rat astrocytes in culture: effect of the ratio of bilirubin to serum albumin. Clin Chim Acta 1996;256:103-14.
- Dennery PA, Seidman DS, Stevenson DK. Neonatal hyperbilirubinemia. N Engl J Med 2001; 344:581-90.
- Dennery PA. Pharmacological interventions for the treatment of neonatal jaundice. Semin Neonatol 2002; 7:111-9.
- Gottstein R, Cooke RI. Systematic review of intravenous immunoglobulin in haemolytic disease of the newborn. Arch Dis Child Fetal Neonatal Ed. 2003;88:F6-10.
- Ip S, Glicken S, Kulig J, O’Brien R, Sege R. Management of Neonatal Hyperbilirubinemia. Summary. Evidence Report/Technology Assessment: Number 65. AHRQ Publication No. 03-E005, March 2002. Agency for Healthcare Research and Quality, Rockville, MD.
- Kaplan M, Hammerman C, and Maisels MJ. Bilirubin Genetics for the Nongeneticist: Hereditary Defects of Neonatal Bilirubin Conjugation. Pediatrics 2003; 111: 886-893.
- Kennan WJ, Noval KK, Sutherland JM, Bryla DA, Fetterly KL. Morbidity and mortality associated with exchange transfusion. Pediatrics 1985; 75(Suppl 2, Pt 2):417-21.
- Lauer BJ, Spector ND. Hyperbilirubinemia in the newborn. Pediatrics in Review 2011;32(8):341-49.
- Maisels MJ, Kring E. Transcutaneous bilirubin levels in the first 96 hours in a normal newborn population of > 35 weeks’ gestation. Pediatrics 2006;117:1169-73.
- Martin JR. A double catheter technique for exchange transfusion in the newborn infant. N. Z. Med J. 1973;77:167-9.
- Martinez JC, Garcia HO, Otheguy LE, Drummond GS, Kappas A. Control of severe hyperbilirubinaemia in full-term newborns with the inhibitor of bilirubin production Sn-Mesoporphyrin. Pediatrics 1999;103:1-5.
- Mehta S, Kumar P, Narang A. Randomized controlled trial of fluid supplementation in term neonates with severe hyperbilirubinemia. J Pediatr 2005;147:781-8.
- Oakden WK, Moore AM, Blaser S, Noseworthy MD. 1H MR spectroscopic characteristics of kernicterus: a possible metabolic signature. AJNR Amer J Neuroradiol 2005; 26:1571-4.
- Pan DH, Rivas Y. Jaundice: Newborn to age 2 months. Pediatrics in Review 2017;38(12):499-577.
- Perlman MA. The late clinical syndrome of posticteric encephalopathy. Pediatr Clin Nth Amer 1960;7:665-87.
- Sgro M, Campbell D, Shah V. Incidence of severe neonatal hyperbilirubinemia in Canada. CMAJ 2006;175:587-90.
- Sgro M, Campbell DM, Kandasamy S, Shah V. Incidence of chronic bilirubin encephalopathy in Canada, 2007-08. Pediatrics 2012;130:886-890.
