Heart & Circulation Disorders
Dr Conall Thomas Morgan, MD, Dr Aideen Moore, MD, FRCPC, MRCPI, MHSc
The material presented here was first published in the Residents’ Handbook of Neonatology, 3rd edition, and is reproduced here with permission from PMPH USA, Ltd. of New Haven, Connecticut and Cary, North Carolina.
Measurement of blood pressure
In the smallest and the sickest infants, blood pressure (BP) is most commonly measured intra-arterially via umbilical or peripheral catheters. If heparin is used in the infusion solution, ensure that total dosage is not higher than 1.0 to 2.0 µ/kg/h. The site of measurement is critical for the interpretation of the values since peripherally placed arterial catheters can result in measurements of systolic pressure as much as 20 mm Hg higher than central ones (1).
The non-invasive oscillometric technique is most commonly used. It results in systolic and diastolic measurements that correlate well with intra-arterial ones (1,2). Oscillometry is convenient, especially when recorded onto the same cardiorespiratory monitoring apparatus. In contrast, some centres still advocate measuring systolic pressures by the Doppler probe technique of de Swiet (3).
Another approach frequently adopted is that the BP is said to be correct when the Mean Arterial BP is at or below the infant’s GA in weeks. While frequently adopted clinically, this has little backing in validity.
Implications for Clinical Practice
- Whichever method is used for non-invasive measures, pay attention to clinical evidence of tissue and peripheral perfusion and not to the values alone.
- State of alertness of the infant and wrong cuff size (cuff width should be about half of the limb circumference (6) can significantly alter the blood pressure.
- When using direct invasive measures, always pay attention to the curve wave-from in case false low readings result from a dampened trace.
“Normal” arterial blood pressure
Owing to the variation in patients, methods, conditions, and ages of measurement, collation of published data to provide normative blood pressures for neonates of all gestational and postnatal ages is difficult. The available published data indicate the following trends:
- a tendency for the blood pressure to rise over the first hours of life;
- Blood pressure if dependent on gestational age. Gestational age (GA) is only marginally better as a predictor of systolic BP than weight, if GA is accurately known;
- at a post-conceptional age of 46 weeks, the blood pressure reaches levels that remain stable during the first year of life.
Two recent normative blood pressures ranges have been published.
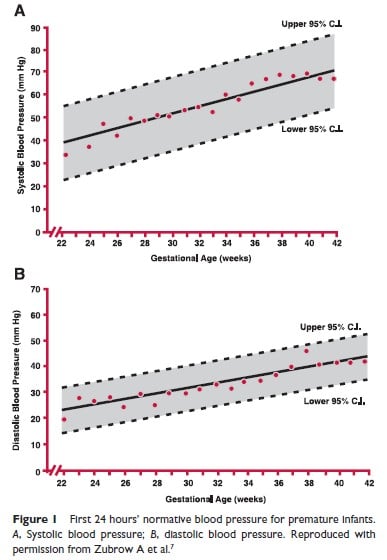
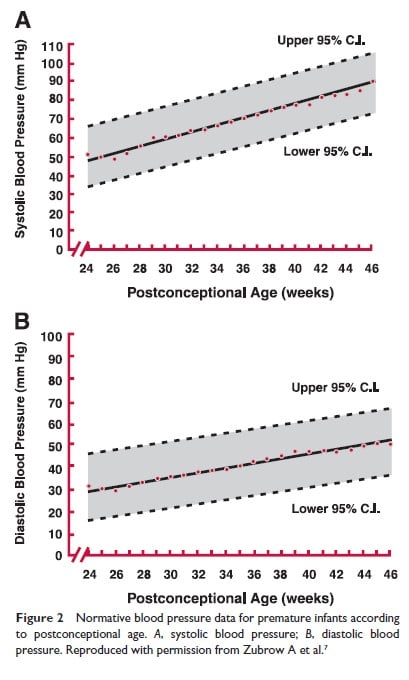
Zubrow and colleagues (7) report on data obtained from premature infants of different gestational ages at 8 hourly intervals from admission to discharge. Figure 1 depicts systolic and diastolic blood pressure averages over the first 24 hours of life in infants from 750 grams to 4000 grams. Figure 2 depicts similar values expressed as a function of postconceptional age up to 46 weeks. For those familiar with using mean BP, these graphs can be useful to derive this value.
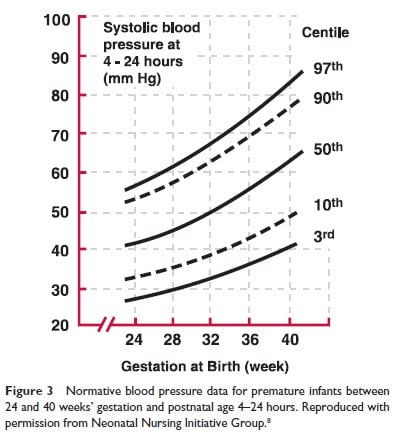
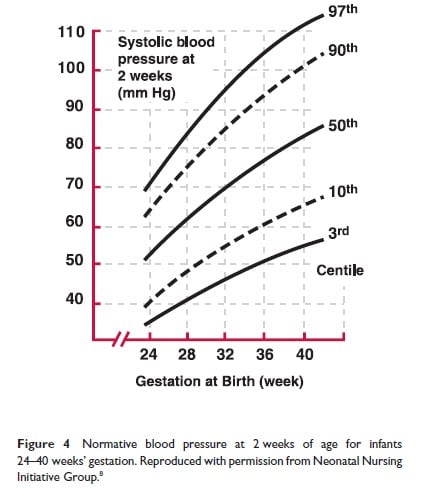
The second study, The Northern Neonatal Nursing Initiative in the UK (8), was performed in a population of infants <32 weeks GA in Northern England from 1990 to 1991; measurements were obtained daily for the first 10 days of life in all infants and further data was collected in a subgroup during the first year of life. Figures 3 and 4 depict, respectively, the BP as obtained at 4 to 24 hours of age in infants between 24 and 40 weeks GA and the 3rd and 97th BP centiles at 2 weeks of age, for the same cohort.
HYPOTENSION
While the ‘normal’ value curves above give some indication of ranges, it is unclear what and when to treat for either high or low blood pressure. This at least in part, explains the great variation in between NICU’s in the incidence of ‘hypotension’ and the corresponding need for drug based therapy (9).
When clinical intervention was deemed necessary in one unit, survivors of hypotension had an increased incidence of mortality, morbidity, and long term motor delay and hearing loss (10).
Given the uncertainty of definitions of hypotension – do not rely solely upon ‘numbers’. While an apparently low BP may cause alarm, it may not be of itself ‘pathologic’ demanding therapy. If tissue oxygenation is truly impaired, anerobic respiration will eventually lead to acidosis.
Consider whether or not there is good tissue perfusion clinically; if after the first 24 hours – has there been adequate urine output? Is there evidence of poor cardiac output with possible tachycardia, or poor tissue oxygenation reflected in acid-base relations with lactic acid production. In these cases, possibly shock has been established. Early intervention will aim to stave off established shock, but this may not always be possible.
Shock
Classification
Reduction in preload
- Hemorrhage
- Transplacental (fetal–maternal) or fetal–fetal (twin-twin) transfusion
- Internal bleeding (eg. subaponeurotic bleeds, bowel perforations; only rarely in intraventricular hemorrhage)
- Traumatic – intra-abdominal; subcapsular hepatic bleed
- Massive pulmonary hemorrhage
- Disseminated intra-vascular coagualtion
- Acute plasma loss
- “Third spacing” in sepsis, necrotizing enterocolitis
- Acute water loss
- Vomiting and/or diarrhea
- Severe evaporative loss
- Excessive use of diuretics
- Intrathoracic compression constraining venous return
- Tension pneumothorax
- Pulmonary interstitial emphysema
- Overventilation of a compliant lung
- Inadvertent PEEP
Reduction in afterload
- Sepsis
- Drugs (eg, tolazoline)
- Hyperthermia
Cardiogenic shock
- Myocardial ischemia
- Acute myocarditis
- Metabolic disturbances (hypoglycemia, hypocalcemia)
- Arrhythmia
- Cardiac tamponade (eg, tension pneumopericardium)
- Left ventricular outflow tract obstruction lesions (hypoplastic left heart syndrome, critical aortic stenosis, coarctation, interrupted aortic arch) Cardiomyopathy
Rare
Endocrine abnormalities
adrenal hemorrhage if very severe
adrenogenital metabolic disorders
Diagnosis
Physical examination findings of shock can be difficult to ascertain early (11).
- Low blood pressure (may be a late sign)
- Thready and rapid pulse
- Prolonged capillary refill time (>3 s)
- Cool extremities (11-12)
- Reduced urine output (output is normally low in the first 24 hours after birth)
- Tachypnea
- Evidence of specific organ dysfunction
Additional Investigations
Arterial blood gas
- Metabolic acidosis
- Often obstinate hypoxemia
Chest radiograph
The size of the cardiac silhouette may provide information regarding the heart size. In cardiogenic shock there may be cardiomegaly.
Echocardiography
While the correlation of ‘normal blood pressure;’ with cardiac output measured by left ventricular output is weak (13) an echo can assist by outruling left ventricular outflow tract lesions, determine ventricular function and may assist by determining adequacy of systemic return using superior vena caval flow (14).
Central venous pressure
The central venous pressure (CVP) is readily measured if an umbilical venous catheter, can be placed above the diaphragm, at the inferior vena cava and right atrial junction.. Pressure transducers placed to observe a normal venous wave-form at the time of insertion of the fluid-filled catheter may assist in positioning. Other sites for CVP measures in newborns, certainly in preterms are not often used.
In reduced pre-load, the venous pressure is low (<5 cm H2O) whereas in cardiogenic shock, venous pressure is high (>8 cm H2O) or normal.
Central venous mixed PO2
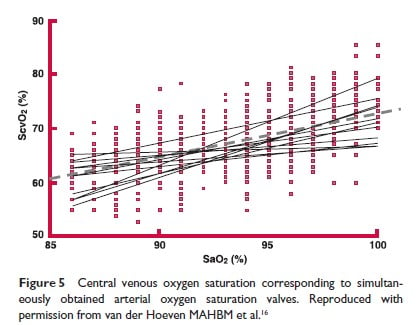
If a central venous pressure catheter is in place, the measurement of the venous PO2 may be used to indirectly assess adequacy of the cardiac output since this parameter is very difficult to measure in the neonate. A decrease in cardiac output, will result in a lower mixed venous P02; reverting to normal values with therapy, will provide indirect evidence of restored cardiac output adequacy. Widespread use of this as a measure of “adequate oxygenation”(15) is discouraged both by practicalities of access and the lack of good validations. However, below are some normative data. As a guide, for normal arterial PaO2, the expected venous PO2 is 40 torr. Alternatively, a range of normal values for central venous O2 saturations is provided in Figure 5 (16). However, to obtain this value of ScvO2, the laboratory must be asked to measure this by a co-oximeter function, on the new generation of blood gas analyzers being used; a calculated value cannot be substituted.
Management
Initial general management should be focused on ensuring vascular access and adequate oxygenation and ventilation.
If necessary, pressure ventilation should be instituted early in shock rather than late. Note that a randomized trial of plasma protein fraction versus dopamine in hypotensive very-low-birth-weight infants showed that early use of dopamine in doses of 10µg/kg/min was more effective than volume, for BP (17). However, volume is still usually the first line in the absence of cardiogenic shock.
If a presumptive diagnosis is made of hypovolemia the first line of therapy is volume. Often a CVP is not immediately available therefore clinical judgment will be necessary.
- In cardiogenic shock, inotropic support will be required as supportive therapy. However mixed pictures occur such that myocardial dysfunction often accompanies shock. Thus, careful consideration ought to be given as to whether inotropes should accompany volume. Consultation with a cardiologist may be of benefit in this circumstance.
Distinguish between ‘early treatment’ and ‘prophylaxis’: a large RCT has shown that so-called “routine” or “prophylactic” expansion of blood volume at birth in VLBW infants is of no benefit to either mortality or to function in 2-year survivors (18).
Volume Expansion
Before use of drug therapy is considered, first attempt volume expansion.
Volumes are for crystalloid 15-20 ml/kg over 15-30 minutes if urgent or over 1-2 hours if less urgent; or colloids and blood between 10-15 ml/kg over 15-30 minutes if urgent.
However, thoughtless over-volume may increase adverse outcomes (19-20).
The most commonly used agents are listed below:
- Crystalloid solutions
- Normal saline
- Ringer’s lactate
- Colloid solutions
- 5% albumin
- Fresh frozen plasma
- Blood – this may be O-negative in emergency situations (especially in delivery room)
Acute hemorrhage should be replaced with blood.
Only limited data are available to guide the choice of crystalloid or colloid solutions but two randomized trials indicates saline is as effective as albumin (21-22). As fresh frozen plasma has associated infective risks (eg, transfusion associated hepatitis, HIV, and cytomegalovirus infections), 5% albumin is preferable. Although plasma does contain coagulation factors, its use may not efficiently restore coagulation activity without considerable volumes (23). When using any of the volume expanders listed, an initial dose of 10 mL/kg infused is recommended. If a reliable measurement of CVP is available, this dose can be repeated until the value is normalized. In its absence, only repeat this volume expansion regimen twice before considering the addition of inotropes to treat possible associated myocardial dysfunction.
Vasocontrictors and Inotropic Agents
Inotropes are indicated for cardiogenic shock, and are also rather broadly use for blood pressure support.
Vasoconstrictor drugs are best aimed at correcting peripheral vasodilation often due to sepsis.
The most common agents are listed below with the recommended dosing, see also a recent overview (25).
Adrenergic Agonists
Dopamine. This is the most commonly used inotropic agent in neonates. It acts through three receptors:
- Dopaminergic (renal vasodilator):2 to 5 µg/kg/min
- ß1-adrenergic (increases cardiac output):5 to 10 µg/kg/min
- α-adrenergic (vasoconstrictor) 10 to 20 µg/kg/min
The presence of receptors is certain after 24 weeks but the demarcation of site-specific actions may be blurred in preterms. This leads some to advocate increases in dose of dopamine beyond these recommended ranges to maintain the BP. This arises especially in some cases of PPHN, where limiting the right-to-left shunt by a higher systemic pressure assists oxygenation. In contrast, others would argue here for a strategy using epinephrine, if dopamine at levels of 20 µg/kg/min is insufficient, as this will also increase inotropic activity of the right ventricle favoring a decreased right atrial pressure.
Dobutamine. A dopamine analog with no selective renal effects and predominantly ß1 effect. The usual dosage is 5 to 20 µg/kg/min. Although a very effective inotrope in older children and adults, its effect in neonates is quite modest, probably because of immaturity of its myocardial receptors (26). Several randomized trials have found dopamine to be more effective than dobutamine in the maintenance of blood pressure in preterms (27). However, cardiac output, and SVC flow is enhanced more by dobutamine (25).
Norepinephrine. This drug has α- and ß1-receptor activity resulting in positive inotropy effects as well as peripheral vasoconstriction. The usual dose is 0.05 to 1 µg/kg/min.
Epinephrine. This agent acts upon α-, ß1-, and ß2-receptors, resulting in positive inotropic and chronotropic activity as well as increased peripheral vascular resistance. The usual dose is 0.05 to 1.5 µg/kg/min.
Phenylephrine. Only α-receptor stimulation is seen with this drug leading to vasoconstriction. This should only be used if the cause of hypotension is solely related to afterload reduction. The usual dose is 0.05 to 1 mg/kg/min.
Isoproterenol. This drug has only ß1– and ß2-receptor activity, thus exhibiting positive chronotropic effects as well as positive inotropy. The usual dosage is 0.05 to 1.0 µg/kg/min (maximum 1.0 µg/kg/min). Discontinue if the heart rate is persistently >200 beats/min.
Phosphodiesterase inhibitors
Milrinone inhibits the action of the phosphodiesterase enzyme 3 which leads to accumulation of cyclic AMP. This results in positive inotropic effects, lusitropic effects (improves diastolic function) as well as decreasing peripheral vascular resistance. Following its proven benefit in infants post-cardiac surgery (28), milrinone has received attention, particularly for use in persistent pulmonary hypertension in newborns (29). It use in preterm infants is also, quite unclear at this time (30).
Sildenafil inhibits the action of the phosphodiesterase enzyme 5 leading to accumulation of cyclic GMP. This results in a decrease in the pulmonary vascular resistance. Sildenfil is not superior to inhaled nitric oxide in the acute management of PPHN but may be of benefit in situations where nitric oxide may not be readily available (31).
Corticosteroids and Vasopressor Resistance
When pressors do not assist, there is a tendency to resort to steroids. Hydrocortisone at 2.5 mg/kg in an RCT performed superior to dopamine (32). There remains a controversy as to whether normal preterm infants are in a state of corticosteroid deficiency (33). A routine supplementation (of dexamethasone at 0.15 mg/kg BW for 3 days) was not supported by randomised trials which found a high rate of acute adverse effects (34). However, in infants with established hypotension refractory to pressors, a lower dose of hydrocortisone, but given over a longer period (1 mg/kg times 8 hourly for 5 days) stabilised BP with less total volume, and pressor support (35-36).
Summary General Approach to Hypotension
Consider postnatal age and gestational age specific blood pressure standards when assessing and treating hypotension. Accurate clinical assessment may be supplemented with arterial blood gas analysis, lactate, CVP and echocardiography. Urine output may not be a reliable measurement within the first 24 hours of life. Consider volume repletion initially followed by inotropic therapy if hypotension and systemic hypoperfusion persists. Consider dopamine or epinephrine if pharmacologic support is required. Consider multiple inotropic agents if hypotension persists or greater peripheral vasoconstriction is needed. Stop any of these drugs if heart rate persists at >200 beats/min. If stability is not achieved, the addition of hydrocortisone may be beneficial. Wean any of the above agents gradually while monitoring the blood pressure and CVP. .. Never use the umbilical artery catheter for vasopressor infusion.
References
- Adelman RD. The hypertensive neonate. Clin Perinatol 1988;15:567-85.
- Frieson RH, et al. Indirect measurement of blood pressure in neonates and infants utilizing an automatic non-invasive oscillometric monitor. Aneth Analg 1981;60:742-5.
- Wareham HA, Haugh LD, Yeager SB, Horbar JD. Prediction of arterial blod pressure in the premature neonate using the oscillometric method. Am J Dis Child 1987;141:1108-10.
- Diprose GK, et al. Dinamap fails to detect hypotensionin very low birth weight infants. Arch Dis Child 1986;61:771-3.
- de Swiet M, et al. Sytolic blood pressure in the first six weeks of life. Arch Dis Child 1980;55:755-7.
- Lum LG, Jone MD. The effect of cuff width on systolic blood pressure measurements in neonates. J Pediatr 1977;91:963-6.
- Zubrow, Hulman S, Kushner H, et al. Determinants of blood pressure in infants admitted to neonatal intensive care units: a prospective multicenter study. J Perinatol 1995;15:470-9.
- Northern Neonatal Nursing Initiative. Systolic BP in babies of less than 32 weeks gestation in the first year of life. Arch Dis Child Fetal Neonatal Ed 1999;80:F38-42.
- Al-Aweel I, Pursely Dm, Rubin LP, Shaha B, Wiesberger S, Richardson DK. Variations in prevalence of hypotension hypertension and vasopressor use in NICU’s. J Perinatology 2001: 21: 272-8.
- Fanaroff JM, Wilson Costello DE, Newman NS, Monteptit M, Fanaroff AA. Treated hypotension is associated with neonatal morbidity and hearing loss in ELBW. Pediatrics 2006; 117)
- Osborn DA, Evans N, Kluckow M. Clinical detection of low upper body blood flow in very premature infants using blood pressure, capillary refill time, and central-peripheral temperature difference.
Arch Dis Child Fetal Neonatal Ed. 2004 Mar;89(2):F168-73. - Lambert HJ, Baylis PH, Coulthard MG. Central-peripheral temperature difference, blood pressure, and arginine-vasopressin in preterm infants undergoing volume expansion. Arch Dis Child Fetal 1998;78:F43-5.
- Evans N. Relationship between BP and cardiac output in preterm infants requiring mechanical ventilation. J Pediatrics 1996; 129:506-12
- Hunt RW, Evans N, Rieger I, Kluckow M. Low superior vena cava flow and neurodevelopment at 3 years in very preterm infants. JPediatr. 2004 Nov;145(5):588-92.
- Dudell G, Cornish J, Bartlett RH. What constitutes adequate oxygen? Pediatrias 1990;85:39-41.
- Van Der Hoeven MAHBM, Maertzdorf WJ, Bianco CE. Continuous ventral venous oxygen saturation measurements using a fibre optic catheter in newborn infants. Arch Dis Child 1996;74:F177-81.
- Gill A, Weinding AM. Randomized controlled trial of plasma protein fraction versus dopamine in hypotensive very low birth weight infants. Arch Dis Child 1993;69:284-7.
- Neonatal Nursing Initiative Group. Randomized trial of prophylactic early fresh or frozen gelatin or glucose in preterm babies: outcome at 2 years. Lancet 1996;348:229-32.
- Ewer Ak, Tyler W, Francis A, Drinkall P, Gardosi JO. Excessive volume expansion and neo natal death in preterm infants born at 27-28 weeks. Paeditr Perinat Epdiemiol 2003: 17: 180-6.
- Hope P. Pump up the volume? The routine use of earl colloid in very preterm infants. Arch Dis Child 1998;76:F163-5.
- Oca MJ, Nelson M, Donn SM Randomised trial of normal saline versus 5% albumin for the treatment for neonatal hypotension. J Perinatol 2003 23: 473-6.
- So KW, Fok TF, Ng PC, et al. Randomized controlled trial of colloid or crystalloid in hypotensive preterm infants. Arch Dis Child Fetal 1997;76:43-6.
- Martin DJ, Lucas CE, Ledgerwood AM, et al. Fresh frozen plasma supplement to massive red blood cell transfusion. Ann Surg 1995;202:505-11.
- The Use of Albumin and/or colloids in newborns – A meta-analysis. Kirpalani H. Pediatr Crit Care Med. 2001 July;2(suppl.):S14-S20.
- Evans N. Which inotrope for which baby? Arch Dis Child Fetal Neonatal Ed. 2006 May;91(3):F213-20.
- Miall-Allen WV, Whitelaw AG. Response to dopamine and dobutamine in the preterm infant less than 30 weeks gestation. Crit Care Med 1989;17:1166-9.
- Subhedar NV, Shaw NJ. Dopamine versus dobutamine for hypotensive preterminfats. The Cochrane database 2003; doi:10.002/1461858, Issue 3 Cd001242
- Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, Chang AC, Bailey JM, Akbary A, Kocsis JF, Kaczmarek R, Spray TL, Wessel DL. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003 Feb 25;107(7):996-1002
- Bassler D, Kreutzer K, McNamara P, Kirpalani H. Milrinone for persistent pulmonary hypertension of the newborn. Cochrane Database Syst Rev. 2010 Nov 10;(11):CD007802. doi: 10.1002/14651858.CD007802.pub2.
- Barrington KJ, Dempsey EM. Cardiovascular support in the preterm: treatments in search of indications. J Pediatr. 2006 Mar;148(3):289-91
- Kelly LE, Ohlsson A, Shah PS. Sildenafil for pulmonary hypertension in neonates. Cochrane Database Syst Rev. 2017 Aug 4;8:CD005494. doi: 10.1002/14651858.CD005494.pub4.
- Bourchier D, Weston PJ. Randomised trial of dopamine compared with hydrocortisone for the treatment of hypotensive very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 1997 May;76(3):F174-8.
- Watterberg KL, Gerdes JS, Cole CH, Aucott SW, Thilo EH, Mammel MC, Couser RJ, Garland JS, Rozycki HJ, Leach CL, Backstrom C, Shaffer ML. Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Pediatrics. 2004 Dec;114(6):1649-57.
- Stark AR, Carlo WA, Tyson JE, Papile LA, Wright LL, Shankaran S, Donovan EF, Oh W, Bauer CR, Saha S, Poole WK, Stoll BJ; National Institute of Child Health and Human Development Neonatal Research Network. Adverse effects of early dexamethasone in extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network.
- Ng PC, Lee CH, Bnur FL, Chan IH, Lee AW, Wong E, Chan HB, Lam CW, Lee BS, Fok TF. A double-blind, randomized, controlled study of a “stress dose” of hydrocortisone for rescue treatment of refractory hypotension in preterm infants. Pediatrics. 2006 Feb;117(2):367-75.
- Seri I. Hydrocortisone is effective in treatment of vasopressor-resistant hypotension in very low birth weight neonates. J Pediatr. 2006 Sep;149(3):422-3.AVRT, AVNRT, AVN, SVT, PJRT, tachycardia, patent ductus
NEONATAL HYPERTENSION
Hypertension is defined as a sustained blood, with a pressure systolic blood pressure greater than 95th percentile for age, size and gender (1,2,3). Use the normative graphs as discussed above.
Hypertension is also sometimes defined by a single cut-off – as a blood pressure > 90/60 mmHg in term infants and >80/50 mmHg in preterm infants during the first 6 weeks of life (4)
Incidence:
0.2%- 3% (5,3, 5A).
Of high risk infants, 9% of babies who had in dwelling umbilical arterial catheter (particularly in high position) developed hypertension.
Clinical Presentation
(1) Routine monitoring: will detect some of the at-risk infants. But since hypertension is very unusual in healthy term newborns, checking blood pressure is not routine. Another related part of the normal newborn exam should however be routine: the palpation of the femoral pulse and ruling out radial-femoral delay.
For infants admitted to NICU, blood pressure must be taken and evaluated in context with the infant’s condition. The onset is usually slow and frequent monitoring of sick at risk infants will detect a trend.
(2) Non-specific insidious symptoms: there cover the gamut of symptoms, and include: feeding difficulties, tachypnoea with no evidence of sepsis or acute respiratory distress, apnoea, Lethargy, irritability and if a very late presentation seizure. Sometimes a chronic failure to thrive in infants discharged from hospital may be present. Sometimes hematuria will start a search for hypotension.
(3) Acute presentation: this may take the form of a cardiovascular collapse with congestive heart failure and cardiogenic shock. Usually in these there may be sudden closure of a duct in a duct-dependent coarctation, or occasionally aortic occlusion-thrombus.
Seizures rarely can signal an intracranial hypertensive crisis that may also include retinopathy.
An endocrine abnormality may be signaled by an acute electrolyte imbalance such as persistent hypokalemia which should precipitate examination for corroborating signs including Ambiguous genitalia.
Historical and Examination clues to diagnosis
- Obtain history pertaining to prenatal exposure (eg Cocaine), NICU course and concurrent clinical condition, medications (pancuronium, vasopressors, steroids), procedures especially umbilical arterial catheterization; history of antenatal rubella exposure.
- Perform a 4-limb BP to evaluate for the presence of aortic arch obstruction.
- Examine for:
Dysmorphic features
Murmur
Absent or diminished femoral pulses
Retinal hypertensive changes
Ambiguous genitalia (congenital adrenal hyperplasia) and excess pigmentation
Abdominal mass (Wilm’s tumour neuroblastoma, polycsytic renal disease
Renal artery stenosis – listen for an abdominal bruit
Etiology: In order of frequency
- Renovascular – by far the commonest cause
Thromboembolism, Renal artery stenosis (rubella infection), Renal venous thrombosis, Mid-aortic coarctation, Compression of renal artery (by tumour or by severe hydronephrosis), Idiopathic arterial calcification - Renal parenchymal disease
Congenital
Polycystic kidney disease, Multicystic –dysplastic kidney disease, Tuberculosis sclerosis, Ureteropelvic junction obstruction, Unilateral renal hypoplasia,Congenital nephritic syndrome
Acquired
Acute tubular necrosis, Cortical necrosis, Interstitial nephritis, Obstruction (stones, tumors)
- Medications/intoxications
Infant
Iatrogenic fluid overload.
Dexamethasone, Adrenergic agents, dopamine, pharmacologic paralytic agents eg pancuronium – rarely caffeine or aminophylline, or opthlamic medicatiosn with pheynylephrine
Maternal
Cocaine. Heroin
- Cardiac
Coarctation of the aorta, interrupted aortic arch - Endocrine
Congenital adrenal hyperplasia, Hyperaldosteronism, Hyperthyroidism - Neurologic
Pain (Post-op period), Intracranial hypertension, Seizures, Subdural hematoma - Neoplasia
Wilms tumor, Mesoblastic nephroma, Neuroblastoma, Pheocromocytoma - Miscellaneous
Total parenteral nutrition, Closure of abdominal wall defect, Adrenal hemorrhage
Hypercalcemia, ECMO
Post-op repaired coarctation, Immediate post-op phaeocromocytoma, Thyroid crisis
Drug withdrawal, Essential HTN (extremely rare)
The most common causes are reno-vascular and renal parenchymal diseases. More specifically, umbilical artery catheter-associated thrombo-embolism affecting either the aorta and/ or the renal arteries accounts for the majority of cases of hypertension in the typical NICU.
Investigations
Urinanalysis (+culture), CBC and platelet, Electrolytes, BUN, creatinine,
Calcium, Thyroid studies
Chest X-Ray, Renal ultrasound with doppler
In selected infants
Echocardiogram
Abdominal/pelvic ultrasound
Urine VMA/HVA, Cortisol, Plasma renin, Plasma aldosterone, VCUG
Aortography, Renal angiography, Nuclear scan (DTPA/Mag-3)
Treatment
In acutely ill infants, assess airway, breathing and circulation, and consider need for urgent intravenous therapy (see below).
Otherwise progress as follows:
- Prior to embarking on therapy, easily correctible iatrogenic causes should be addressed:
Remove UA catheter, consider all drugs and if possible wean or stop. Calculate all fluids received in prior 24-48 hours. - Therapy should be tailored to the clinical condition and severity of the hypertension.
- Avoid too rapid reduction in blood pressure to avoid cerebral ischemia and hemorrhage.
- Aim to decrease the blood pressure by one third of the desired total in the first 6 hours
- Monitor blood pressure continuously or frequently by repeated cuff readings.
Emergency Drug Management of Blood Pressure
First Line Medications:
Hydralazine, 0.15-0.6 mg/kg/dose or 0.75-5 mcg/kg/min IV bolus or constant infusion.
Second Line: Other Specific Medications for Hypertension:
The following are the large number of possibilities.
We would advise using hydralazine and or Diazoxide is used as the main second line; it is very fast-acting and can be used in true crisis situations.
Both nifedipine and nitroprusside are not used commonly in infants.
Additional Agents:
Use of beta –blockade with propanolol type agents – should be used with care since they may impede cardiac output.
Table 1. Intravenous Drugs for Severe Hypertension in Neonates
| Drug | Class | Dosage | Comments |
| Diazoxide | Vasodilator (arteriolar) | 2-5 mg/kg/dose rapid IV bolus | Should be given as rapid bolus as slow IV injection ineffective; duration unpredictable;use with caution, may cause rapid hypotension,hypoglycemia |
| Esmolol | Beta-blocker | 100-300 mcg/kg/min IV infusion | Very short-acting; constant IV infusion necessary, may cause hypotension |
| Hydralazine | Vasodilator (arteriolar) | 0.15-0.6 mg/kg/dose or 0.75-5 mcg/kg/min IV bolus or constant infusion | Tachycardia is frequent adverse effect; must administer q4h with IV bolus |
| Labetalol | Alpha- and beta-blocker | 0.2-1 mg/kg/dose or 0.25-3 mg/kg/h IV bolus or constant infusion | Heart failure, BPD relative contraindications |
(modified from J Flynn, 2004)
Seizures may be treated with slow IV infusion of Phenobarbitone or Diazepam.
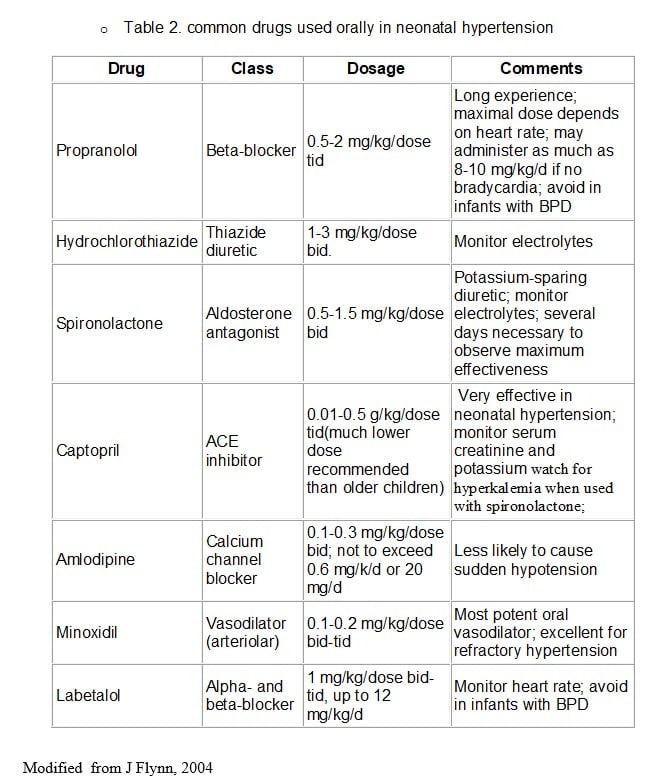
Drug Therapy in a more Stable setting – Oral therapy
Less severe cases or whose acute hypertension has been controlled and ready to be transitioned to chronic therapy Modified from J Flynn, 2004
Surgery
Obviously directed at the cause
- It is rare, but unilateral nephrectomy may be needed to prevent a so-called ‘Goldblatt kidney”, where reversible hypertension becomes ‘fixed.
- Cardiac lesions will need urgent correction if they are the cause
- Thrombectomy and anticoagulation is discussed in the hematology chapter.
Indications:
Cardiac: Coarctation of aorta, aortic obstruction with thrombus
Renal: Ureteral obstruction,Wilm’s tumour, neuroblastoma, Polycystic kidney, Pheocromocytoma, PUJ obstruction, Renal vein thrombosis
Outcome
Long term prognosis depends on the underlying etiology of hypertension.
- UA catheter –will resolve slowly overtime.
- Renal parenchymal diseases may be present throughout childhood.
- Renal artery stenosis repair- persistent or late HT
- Coarctation generally has excellent long term outcomes following neonatal repair.
References:
- Tank, Clin Pediatrn.1987; 26:21-24,
- Rajpoot D. J Perinatal 1999:19(8) 532-83,
- Flynn I. Pediatr Nephrol 2000; 14:332 -341.
- Wareham IA, Haugh LD, Yeager SB, Horbar JD, Am J Dis Child 1987;141:1103-10
- Rajpoot D. J Perinatal 1999:19(8) 532-83.
- Fanaroff JM and Fanaroff AA Blood pressure disorders in the neonate: hypotension and hypertension. Seminars Fetal & Neonatal Medicine 2006:11; 174-181.
- Watkinson M. Hypertension in the newborn baby. Arch Dis Child Fetal Neonatal Ed 2002; 86:F78-F81.
- Skalina MEL, Kliegman RM, Fanaroff AA, Am J Perinatol 1986:3:235-239,
- Singh HP, Hurley RM ,Myers TF. Neonatal hypertension.Incidence and risk factors. Am J Hypertens 1992, 5(2):51-55).
- Abman SH, Warady BA, Lum GM, Koops BL J Pediatr 1984; 104:929-31
- Alagappan A, Malloy MH Am J perinatol 1998 ;15 :3-8).
- Boedy RF, Goldberg AK, Howell CG Jr, Hulse E, Edwards EG, Kanto WP. 1990 J Pediatr Surg; 25:258-61
- Sell LL, Cullen ML, Lerner GR, Shanley CJ, Klein MD Surgery; 102: 724-730
PATENT DUCTUS ARTERIOSUS
Incidence
- While in most term infants the ductus constricts spontaneously during the first 24 hours after birth, this does not occur in preterms. In preterm infants, the duct may reopen after functional closure owing to reduced oxygen or increased circulating prostaglandin as occurs in asphyxial states or pulmonary disease, such as aspiration pneumonia1.
- Incidence of patent ductus arteriosus (PDA) varies anywhere between 10-60 % depending on the gestational age (lower the gestational age, higher the incidence) and the day of life 2.
- ‘Asymptomatic PDA’ can occur in 50% of infants with birth weight less than 1500 gms3
- Different strategies for fluid balance, ventilatory support, drug therapy- all account for wide variation of reported incidences.
Diagnosis
Significant PDA can be diagnosed clinically, but sensitivity and specificity of all the following clinical and radiological signs are poor, making echocardiography the gold standard 4.
Clinical:
- Tachycardia
- Systolic or continuous murmur; occasionally a large duct may be silent.
- Hyperactive precordium
- Bounding peripheral pulses – in small preterms the palmar pulse or pedal pulse should be impalpable and if felt implies volume loading.
- Wide pulse pressure (>25 mm Hg)
- Increasing ventilatory requirements and/or ventilator dependency
- Pulmonary hemorrhage (manifests as pink frothy aspirates).
Differential:
Signs of left to right shunt are often difficult to tease out from pulmonary disease or sepsis.
Radiological/ Chest X Ray: Cardiomegaly with increased pulmonary vascularity.
Ideally, diagnosis of patent ductus arteriosus should always be confirmed by echocardiography before treatment for following reasons:
- The shunt may be trivial and hemodynamically insignificant.
- Undiagnosed ductal dependent congenital heart lesions may be present,
Echocardiography: Significant PDA is diagnosed when:
- Ductal diameter >1.5 mm per kilogram body weight
- Left atrial and left ventricular enlargement
- Left atrium /aortic root diameter ratio>1.4
- Holodiastolic flow reversal in the descending aorta
- Dilated branch pulmonary arteries with antegrade diastolic flow
Clinical Burden from PDA
- The contribution of PDA to long term outcome has been much debated, but early closure with prophylaxis by indomethacin does not appear to confer clinical benefit in terms of reducing BPD, or long term functioning at 18 months5.
- Short term fluctuations in velocities of cerebral blood flow may cause IVH, but do not appear to affect long term outcome
- The reverse end diastolic flow aortic blood flow (diastolic steal) associated with PDA may cause renal impairment, intestinal ischemia with NEC and gastrointestinal perforation, and reduced middle cerebral artery blood flow velocity.6,7
- Given the uncertainties of benefit – some have questioned the need for medical closure or ligation pending a large RCT.8 Most clinicians currently, in the absence of more definitive data will try to close a PDA given the inability to wean form a ventilator. This thinking often underlies approach to the recurrent PDA also.3,9
Management
Medical
Cyclooxygenase inhibitors (indomethacin or ibuprofen) have both been approved for the closure of hemodynamically significant PDAs. Various regimens are advocated and they are effective in 70-80% of cases. Paracetamol also decreases prostacyclin synthesis; however, unlike COX inhibitors, it does not have a peripheral vaso-constrictive effect and can be used in infants with contraindications to non-steroidal anti-inflammatory drugs.
The rare side-effect of oliguria is almost invariably short term,
The advocates of ibuprofen have enrolled and followed many fewer infants to long term than those using indomethacin, and its advantages are therefore moot.10
Oral indomethacin therapy is not recommended as it may increase GI perforations.
High dose, short course: Administer 0.2mg/kg/dose in an IV dose followed by 0.1mg/kg every 12 hours for a total of three doses. The interval between the doses can be increased if infant develops oliguria.
Low dose, prolonged course: Administer 0.2mg/kg in an IV dose followed by 0.1mg/kg/dose every 24 hours for a total of 5 doses.
We follow a standard dosing as followed in the TIPP trial.5
Monitoring during indomethacin therapy:
- Urine output: If significant oliguria develops, restrict the fluids
- Drugs excreted by the kidneys (e.g. aminoglycosides, digoxin) – reduce the dose and monitor blood levels if oliguria develops
- Serum electrolytes because of hyponatremia resulting from renal tubular inhibition
Complications of any cyclooxgenase therapy:
- Transient or permanent renal impairment
- Relative hypoxemia.11
- Thrombocytopenia
- Impaired cerebral blood flow.
- Gastrointestinal side effects with perforation and necrotizing enterocolitis
Contraindications:
- Duct dependent cardiac lesions
- Intraventricular hemorrhage, grades III and IV.
- Creatinine >150 mmol/L
- Oliguria (urine output <0.5 ml/kg/h)
- Clinical bleeding, platelets <80,000/cm3 (pulmonary hemorrhage is not an contraindication if temporally related to PDA)
- Necrotizing enterocolitis
As we suggest above, prior cardiac imaging should rule out CHD. But this may not always be possible, and rarely a dependent cardiac lesion will be unmasked. Reversal of indomethacin-induced ductal closure by administration of prostaglandin can be achieved.
Emergency Management of Massive Pulmonary hemorrhage
(also see respiratory section). Given the rare complications of coagulation disorder, indomethacin is not used. Attention to stabilizing cardiovascular stability with volume, high PEEP is most usual. Following this, ligation is usual. MPH carries a poor prognosis.12
Surgical treatment:
Surgical ligation is usually reserved for “medical failures”. The failure of closure rates were reported in between 30-40% of treated infants. The failure of closure rate increases significantly the more the degree of prematurity.3,9 Infants who are <28 weeks of GA and who still had Doppler evidence of ductus luminal flow after completing the initial course of indomethacin are unlikely to respond to subsequent courses of indomethacin.9 However, treatment with 2nd course of indomethacin still remains the treatment of choice if there is a hemodynamically significant PDA. Increasing awareness of operative problems in long term outcome introduce a new caution.8,14,15
Some centers perform surgery in the nursery to avoid added hazards of transport. Some centres have now started performing PDA closure using a transcatheter device under ECHO guidance to eliminate the effects of both radiation and contrast on the preterm infant.
Complications following PDA ligation are rare. They include:
- Inadvertent ligation of the aorta or the left pulmonary artery;
- Thrombus in the great vessels from handling related trauma;
- rupture of the thoracic duct with consequent pleural chylous effusion;
- damage to recurrent laryngeal nerve with vocal cord paralysis
References:
- Weiss H, Cooper B, Brook M, Schlueter M, Clyman R. Factors determining the loss of ductus arteriosus responsiveness to prostaglandin E2. Circulation 1983; 68: 433-6.
- Clyman RL Ontogeny of the ductus arteriosus response to prostaglandins and inhibitors of their synthesis. Seminars in Perinatology 1980 4:115-124.
- Evans N. Current controversies in the diagnosis and treatment of patent ductus arteriosus in preterm infants. Adv Neonatal Care. 2003 Aug 3(4):168-77.
- Davis P, Turner-Gomes S, Cunningham K, Way C, Roberts R, Schmidt B. Precision and accuracy of clinical and radiological signs in premature infants at risk of patent ductus arteriosus. Arch Pediatr Adolesc Med. 1995 Oct;149(10):1136-41.
- B. Schmidt, P. Davis, D. Moddemann, A. Ohlsson, R.S. Roberts and S. Saigal et al., Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants, N Engl J Med 344 (2001) (26), pp. 1966–1972
- Shimada S, Kasai T, Konishi M, Fujiwara T. Effects of PDA on left ventricular output and oxygen blood flow in preterm infants with RDS treated with surfactant. J Pediatr 1994;125: 270-7
- Weir FJ, Ohslon A, Myhr TL, Fong K, Rayan ML. A patent ductus arteriosus is associated with reduced middle cerebral artery blood flow velocity. Eur J Pediatr 1999;158: 484-7
- Bose CL, Laughon M. Treatment to prevent patency of the ductus arteriosus: beneficial or harmful? J Pediatr. 2006 Jun;148(6):713-4.
- Keller RL, Clyman RI. Persistent Doppler flow predicts lack of response to multiple courses of indomethacin in premature infants with recurrent patent ductus arteriosus. Pediatrics. 2003 Sep;112(3 Pt 1):583-7.
- Van Overmeive B,Smets K ,Lecoutere D,Van DeBroek H ,Weyler I,De Groote K. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl I Med 2000;343:674-81.
- Schmidt B, Roberts RS, Fanaroff A, Davis P, Kirpalani HM, Nwaesei C, Vincer M; TIPP Investigators. Indomethacin prophylaxis, patent ductus arteriosus, and the risk of bronchopulmonary dysplasia: further analyses from the Trial of Indomethacin Prophylaxis in Preterms (TIPP). J Pediatr. 2006 Jun;148(6):730-734
- Kluckow M, Evans N. Ductal shunting, high pulmonary blood flow, and pulmonary hemorrhage. J Pediatr. 2000 Jul;137(1):68-7
- Alfaleh K, smyth JA, Roberts RS, Solimano A, Asztalos EV, B.Pediatrics 2008;121(2):e233-8.
- Perez CA, Bustroff Silva IM, Vilasenor E, Fonkalsurd EW, Atkinson IB. Surgical ligation of patent ductus arteriosus in very low birth weight infants: is it safe? Am Surg 1998;64:1007-9.
- Kabra K. NS, Schmidt B, Roberts RS, Doyle LW, Papile L, Fanaroff A. Neurosensory impairment after surgical closure of patent ductus arteriosus in extremely-low-birth-weight infants: results from the Trial of Indomethicin Prophylaxis in Preterms (TIPP). J Pediatr 2007 Mar;150(3):229-34, 234.e1
CARDIAC ARRHYTHMIA AND CONDUCTION ABNORMALITIES
Normal heart rate
The normal heart rates in neonates can vary from 80 to 180 beats/min (24). Episodes of sinus bradycardia (<80 beats/min), however, are fairly frequent in healthy term infants and in preterm infants before discharge. They are most often benign in the absence of apnea or other manifestations of heart disease, especially when present in sleep.
Supraventricular tachycardia
Supraventricular tachycardia (SVT) is the term used to describe arrhythmias originating from the atria. Examples of SVT include; atrioventricular reentrant tachycardia (AVRT), atrioventricular nodal reentrant tachycardia (AVNRT), persistent junctional reciprocating tachycardia (PJRT), atrial flutter, atrial ectopic tachycardia and atrial fibrillation.
Typically SVT’s are narrow complex tachycardia’s but can be wide complex in the following circumstances:
- SVT with preexisting right bundle branch block
- SVT with rate related aberrancy (rate related bundle branch block)
- SVT with preexcitation
- SVT with an antidromic reentrant pathway
The commonest type of SVT occurring in neonates are reentrant tachycardias (AVRT) and atrial flutter.
AVRT can be suspected on ECG by the apparent lack of P waves (can be seen retrograde), tachycardia and lack of heart rate variability. Structural congenital heart disease is uncommon, assessment by echocardiography is warranted to out rule known associations and to establish ventricular function. AVRT typically terminates with adenosine.
AVNRT typically occurs in older children and not in neonates. The presentation and ECG appearances are similar to that of AVRT. AVNRT typically terminates with adenosine.
Atrial flutter is less common than AVRT and can be seen both in fetal and postnatal life, often within the first few days. The ECG has a classic saw tooth appearance due to continuous atrial activation with rates of between 300-600bpm and variable conduction to the ventricles. Atrial flutter does not terminate with adenosine but temporary blockage of the AVN allows diagnosis of the arrhythmia by making the flutter waves easier to appreciate.
PJRT typically presents after the neonatal period and as the name suggests is incessant in about 50%. Classic ECG finding of a long RP tachycardia (therefore similar to sinus rhythm) is of a negative P wave in the inferior leads (II and III). Usually no response to adenosine.
Atrial ectopic tachycardia is uncommon and is characterized by an abnormal focus in the atria that depolarizes faster than the AVN. The ECG is characterized by differing P wave morphologies and axis and exhibits ‘automaticity’. Automaticity is influenced by sympathetic drive (fever, pain, anxiety) and leads to a gradual warm up and cooling down of the arrhythmia. AET does not terminate with adenosine.
Wolf Parkinson White (WPW)
WPW Syndrome is the term used to describe SVT due to preexcitation. Preexcitation is sometimes evident on the resting ECG and has three key findings; a short PR interval, a delta wave and a prolonged QRS duration. Sometimes there is a concealed pathway resulting in a normal resting ECG.
WPW is associated with congenital heart disease, in particular Ebsteins anomaly and congenitally corrected transposition of the great arteries.
In the rare instance of atrial fibrillation in the setting of WPW, adenosine is contraindicated as there is a risk of inducing ventricular tachycardia/ fibrillation. Preexcitation atrial fibrillation is extremely uncommon in the neonatal and indeed pediatric world, however an irregularly irregular wide complex tachycardia should prompt urgent electrical cardioversion or medical cardioversion with procainamide in consultation with a pediatric cardiologist.
Ventricular tachycardia
Wide complex tachycardias are ventricular in origin until proven otherwise. Heart rate nor hemodynamic condition of the infant are factors when determining whether or not a wide complex tachycardia is ventricular in origin.
Atrioventricular dissociation, fusion beats or sinus capture beats are diagnostic for VT.
VT is rare in neonates and is usually associated with cardiac tumours (rhabdomyomas in tuberous sclerosis), severe electrolyte disturbances, long QT syndrome and myocarditis.
Treatment of Supraventricular tachycardia (See Figure 6)
Fig. 6 Narrow complex regular tachycardia (reentry type SVT)
The hemodynamic condition of the infant will determine the treatment strategy for SVT. Any evidence of hemodynamic compromise should prompt synchronized electrical cardioversion at 0.5-1 J/kg following sedation if time allows.
Hemodynamically stable SVT should be managed as follows:
- Vagal stimulation can be achieved a number of ways including; by applying an icepack to the face; passage of a NG tube; rectal temperature.
- Adenosine at a dose of 0.1mg/kg to a maximum dose of 0.2mg/kg should be given intravenously as close to the heart as possible. This can be achieved by a umbilical venous catheter, a jugular central line or a periperhal cannula in the right antecubital fossa. Adenosine should not be delivered via an intraosseous line. Continuous cardiorespiratory monitoring should be used while delivering adenosine.
- Betablockers are first line pharmacological management of SVT. Oral propranolol is (1mg/kg/BID) used once sinus rhythm is restored. Attention should be given to hypoglycemia which can be observed in the first 48 hours. Should the arrhythmia persist despite vagal stimulation and multiple doses of adenosine or oral medication is contraindicated, intravenous esmolol via a continuous infusion is a good choice to achieve rate control. Esmolol has a very short half-life. A starting dose of between 50-100mcg/kg/min increasing by 50mcg/kg/min every 15 minutes until rate control is achieved is a suggested regime. Once rate control is achieved, an attempt at arrhythmia termination with adenosine should be tried, which is often successful.
Flecainide is a second line pharmacotherapy for the management of SVT. This should not be commenced without the consultation of a pediatric cardiologist. Flecainide can interfere with milk and can cause widening of the QRS therefore daily ECG’s should be performed for the first 72 hours following commencement of the drug.
Most neonatal cases of SVT (due to AVRT/WPW) requiring pharmacotherapy usually are treated for 9-12 months with a low rate of recurrence. Follow up of these patients should be by a pediatric cardiologist. Parent education in auscultation should be provided prior to discharge from the NICU.
Treatment of other arrhythmias
Atrial flutter responds well to DC cardioversion without the need for long term pharmacotherapy. Risk of recurrence is extremely low.
Atrial Ectopic Tachycardia is treated with beat blockers and/or flecainde.
PJRT as the name suggests can be difficult to manage often requiring multiple anti arrhythmic drugs.
Ventricular tachycardia if hemodynamically unstable is managed with electrical cardioversion. Treatment depends on the underlying diagnosis, but often Beta blockers and amiodarone are used both in the acute and chronic management of this arrhythmia
Complete heart block (see Figure 7)
Complete heart block in neonates in the setting of a structurally normal heart is usually caused by in utero transfer of maternal antibodies (Anti Ro/La) due to maternal SLE or Sjogren’s syndrome. Congenital heart lesions associated with complete heart block include congenitally corrected TGA and left atrial isomerism.
Indications for permanent pacemaker placement include
- Heart rate of less than 55bpm (or 70bpm in the setting of CHD)
- Wide ventricular escape rhythm
- Complex ventricular ectopy
- Ventricular dysfunction.
Medical management of complete heart block includes isoproterenol infusion, which has mainly chronotropic effects, to increase the overall heart rate. Epinephrine can be used as a second line agent.
Fig. 7
Congenital Heart Disease
Suspect CHD in infants with the following:
- Cardiac murmurs, though by itself, in first hours of life is a poor sign of CHD
- Central cyanosis, ie, arterial hypoxemia, SaO2 < 85%
- Hyperactive precordium
- Arrhythmia, especially PAT with atrial enlargement, and complete heart block
- Upper-limb BP > lower-limb BP by > 20 mm Hg
- Tachypnea, hepatomealy Ascites, or hydrops
The hyperoxia test result is “positive” (ie, suggests CHD) if PaO2 is < 150 mm Hg in FiO2 of 1.00 in a patient without evidence of severe lung disease. If the infant is already ventilated, the test’s predictive accuracy is enhanced by PEEP. Figure 8 is a schematic diagram showing the initial steps for diagnosis in congenital heart disease.
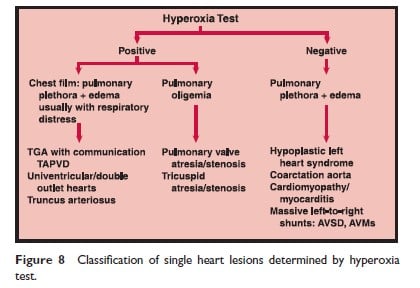
Management
- Mechanical ventilation, if respiratory failure is present
- Diuretics (furosemide, hydrochlorothiazide)
- Intotropes for shock-like state
- Treat metabolic acidosis with bicarbonate or THAM
- Consider PGE1 (see below for indications)
- Treat arrhythmia
____________________________________
Prostaglandin E1 (PGE1)
Indications
Indications include the following:
- Reduced pulmonary perfusion, eg, pulmonary atresia, tricuspid atresia
- Left-sided obstructive lesions including hypoplastic left heart syndrome, coarctation of aorta, and interrupted aortic arch
- Poor mixing. Transposition of the great vessels (but note that in patients with very restrictive interatrial communication, the increase in pulmonary blood flow caused by dilating the PDA may result in pulmonary edema and worsening of the blood gasses)
- Persistent metabolic acidosis and /or severe cyanosis requiring transport (attempt to exclude contraindications)
Contraindications
Contraindications include total anomalous pulmonary venous return with pulmonary venous obstruction, and an infant with unconfirmed diagnosis requiring transport but who is stable and not acidotic from tissue hypoxia.
Dose
Administer Give 0.5-3.0 microgram/Kg per hour µg/kg/min. When SaO2 or PaO2 rises, gradually slow down the infusion rate to lowers effective rate. Higher dosing leads to more apneas, and increases pulmonary flooding with left to right shunt. If PGE1 is not beneficial, discontinue and reconsider diagnosis.
Adverse Reactions
- Apnea
- Hypotension
- Fever, flushing
- Bradycardia or tachycardia
- Seizures
Table 3. Altered Heart Rates
| Cause | Tachycardia | Bradycardia |
| Ambient temperature
Arousal state
Pyrexia
Medications
Endocrine
Hematologic
Cardiovascular |
> thermoneutral range
Pain
Present
Inotropes, xanthines
Hyperthyroidism, hyperammonemia
Anemia
Congestive heart failure Supraventricular tachycardia |
Deep sleep
Propranolol, digoxin
Hypothyroidism
Congenital heart block |
References
- Richards JM, Alexander JM, Alexander JR et al. Sequential 22-hour profiles of breathing patterns and heart rate in 110 full-term infants during their first 6 months of life. Pediatrics 1984;74:763-77.
- Wren C: Cardiac arrhythmias in the fetus and newborn. Semin Fetal Neonatal Med. 2006 Jun;11(3):182-90.
