COVID-19 AND NEONATES
March 16, 2021
Management of COVID-19 Positive Neonates and Mothers
Management for COVID-19 Positive Neonates:
There is ongoing debate surrounding care for neonates who test positive for SARS-CoV-2. Severe neonatal infections with COVID-19 continue to be rare, however, they do occur. Thus, it is vital to have both early and effective treatment for neonatal acute respiratory distress due to COVID-19.
Neonates with Symptomatic COVID-19 Infection:
The majority of symptomatic COVID-19 cases in neonates are managed with supportive measures, which may include mechanical ventilation, oxygen, intravenous fluids and electrolytes or empiric antibiotics, in the case of bacterial superinfection1.
The American College of Obstetricians and Gynecologists currently recommends that antenatal corticosteroids be used if necessary, regardless of SARS-CoV-2 status2. Recent studies have shown that the use of dexamethasone in adult patients with severe COVID-19, requiring mechanical ventilation or oxygen, improved the 28-day mortality rate3. The risks and benefits of using dexamethasone in the paediatric population with COVID-19 are still debated. Dexamethasone should be administered in the paediatric population at a dose of 0.15 mg/kg daily, through oral, intravenous or nasogastric administration4.
Specific anti-virals, including Remdesivir, have been tried in the adult population for the management of COVID-19. Remdesivir is an anti-viral that is well-known for its efficacy in treating the Ebola outbreak that occurred in 2014. Although it has not been tested for its safety or efficacy in terms of treating SARS-CoV-2 infections in neonates, it was used in children as young as 5 days old during the Ebola outbreak5. UpToDate recommends that the Remdesivir dose administered should be determined by the child’s weight. For children > 3.5 kg and < 40 kg, a loading dose of 5 mg/kg IV should be administered. After that, a maintenance dose of 2.5 mg/kg IV should be administered every 24 hours4. Of note, Remdesivir should not be co-administered with hydroxychloroquine and chloroquine, due to drug interactions which decrease the efficacy of Remdesivir. A case study of a neonate with acute respiratory distress due to COVID-19 was treated with 7 doses of Remdesivir, with improvement and stable creatinine and liver function tests6.
Care of neonates born to COVID-19 Positive Mothers:
In terms of breastfeeding, a mother that is positive for SARS-CoV-2 is allowed to breastfeed if she maintains the proper precautions, including hand hygiene and a mask. Alternatively, the mother may express breastmilk which is subsequently fed to the baby by an uninfected caregiver. If an infant born to a SARS-CoV-2 mother requires NICU admission, the baby should be admitted in a single room with negative pressure air. These infants should be tested at 24 hours of life, and again at 48 hours of life. On SARS-CoV-2 positive infants, testing should continue every 48-72 hours until two negative tests are obtained. If the baby is unable to be tested, they should be treated as positive for a 14 day period. The WHO further supports skin-skin contact regardless of infection status1.
COVID-19 Vaccination in Pregnancy:
With vaccination against COVID-19 becoming available, the National Advisory Committee for Immunizations (NACI) allows pregnant and breastfeeding women to receive the option to become vaccinated against COVID-19, but the benefits of vaccination must outweigh the risks. Each patient should be made aware of the lack of clinical vaccine trial in this population7.
References
- Barrero-Castillero, A., Beam, K.S., Bernardini, L.B. et al.COVID-19: neonatal–perinatal perspectives. J Perinatol (2020). https://doi.org/10.1038/s41372-020-00874-x
- COVID-19 FAQs for Obstetrician-Gynecologists, Obstetrics: The American College of Obstetricians and Gynecologists.https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics.
- Recovery Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19—Preliminary Report. N Engl J Med. 2020; epub ahead of print July 2020.https://doi.org/10.1056/NEJMoa2021436
- Deville, J., Song, E. and Ouellette, C., 2021. Uptodate. [online] Uptodate.com. Available at: <https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-management-in-children?topicRef=128190&source=see_link#H1096670095> [Accessed 16 January 2021].
- Mulangu S, Dodd LE, Davey RT Jr., Tshiani Mbaya O, Proschan M, Mukadi D, et al. A randomized, controlled trial of Ebola Virus disease therapeutics. N Engl J Med. 2019;381:2293–303.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7454701/
- https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines.html
Authored by Maria Casalino. Edited by Dr. Ahuva Brown and Dr. Mary Woodward.
February 16, 2021
COVID-19 Reference
Click here to access the comprehensive resource from Dr. Bernd S. Kamps and Dr. Christian Hoffmann summarizing weekly scientific updates on COVID-19, This excellent reference provides information as it becomes available on what is known about the pathogenesis and transmission of the virus, an international timeline of COVID-19, how it presents in both adult and paediatric populations, and much more. Click on specific sub-headings in the Table of Contents, to be re-directed to the relevant section of the text.
February 8, 2021
Summary of the Current COVID-19 Vaccines
mRNA Vaccines Overview
Messenger RNA (mRNA) is a piece of genetic material that contains instructions to direct the cells in your body to produce a specific protein. The mRNA in the vaccine is protected by an outer layer that prevents the vaccine from being broken down by the body. In the case of the COVID-19 vaccines, the mRNA in the vaccine codes for the production of spike protein, a unique feature of SARS-CoV-2 that is highly involved in the pathogenesis of COVID-19. The protein is then transported to the cell surface, where it acts as an antigen. This means that the immune system will detect the foreign protein and produce protective antibodies against it. Upon exposure to SARS-CoV-2 following injection of the vaccine, your body will be equipped with the necessary antibodies to fight off infection.
It is important to note that neither the Pfizer nor Moderna vaccines have been tried in neonatal, paediatric, or pregnant populations.
Reference: https://www.cdc.gov/vaccines/covid-19/hcp/mrna-vaccine-basics.html
Click the arrows below to read more about each vaccine:
Pfizer Vaccine
The full BioNTech & Pfizer Clinical Trial report can be found here: https://www.nejm.org/doi/full/10.1056/NEJMoa2034577
The Pfizer vaccine is an mRNA vaccine which codes for the SARS-CoV-2 spike protein, involved in the pathogenesis and development of COVID-19. A randomized control study involving 43,448 participants was conducted on adults, at least 16-years-old, and did not include children, adolescents, or pregnant women. Participants were healthy with stable chronic conditions, including HIV, hepatitis B and hepatitis C. Participants were excluded from the study if they had a history of COVID-19 infection, were on immunosuppressive therapy or have been diagnosed with an immunosuppressive condition.

Research Summary figure reprinted with permission from Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine. 2020;383(27):2603-15.
The vaccine or a placebo was delivered intramuscularly on 2 occasions, 21 days apart. The study found that two doses of the vaccine elicited high SARS-CoV-2 neutralizing antibody titres.

Research Summary figure reprinted with permission from Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine. 2020;383(27):2603-15.
Efficacy
The study established a vaccine efficacy of 95% (95% confidence interval of 90.3-97.6). Protection against COVID-19 with the vaccine occurs as soon as 12 days after the first dose. Furthermore, when the vaccine was trialled in individuals across subgroups including age, sex, race, ethnicity, obesity, and co-existing conditions, such as hypertension, it was discovered to have the same level in efficacy as the general population
Local effects
Local effects were more common than systemic effects, however both remained rare. Mild to moderate pain at injection site within 7 days of vaccination was the most common local reaction. This was mostly reported among the younger population. A small percentage of participants reported injection-site redness that self-resolved within 1-2 days.
Systemic effects
Systemic effects included fatigue and headache, primarily following the second dose of the vaccine, although these were also reported by the placebo group. A total of 4% of the vaccine participants reported severe fatigue. Younger participants, aged 16-55 years-old, reported these side effects more. The frequency of any severe systemic event occurring after the first dose was 0.9%. Fever following the second dose was reported by 16% of younger vaccine recipients and 11% of older recipients, aged > 55.
Adverse events
Adverse events occurred at a similar rate in both the vaccine and placebo groups at 0.6% and 0.5%, respectively. A total of four related serious adverse events were reported in the vaccine participant group. These included a shoulder injury, right axillary lymphadenopathy, paroxysmal ventricular arrhythmia, and right leg paraesthesia. The lymphadenopathy is likely explained by the vaccine causing an immune response. In addition, 2 participants died in the vaccine group – one from arteriolosclerosis and one from an acute myocardial infarction. In the placebo group, 4 patients died – 2 from unknown causes, one from a haemorrhagic stroke and one from a myocardial infarction. However, no deaths were related to the vaccine or placebo. In addition, there were no COVID-19-related deaths in both the placebo and vaccine groups.
Moderna Vaccine
The full Moderna Clinical Trial Report can be found here: https://www.nejm.org/doi/full/10.1056/NEJMoa2035389
The SARS-CoV-2 vaccine produced by Moderna is a lipid encapsulated mRNA vaccine encoding spike protein as well. A randomized control trial was performed involving 30,420 participants. Two intramuscular injections of either vaccine or placebo were given at 28 days apart. Participants were at least 18 years old with no known history of SARS-CoV-2.

Research Summary figure reprinted with permission from Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. New England Journal of Medicine. 2020;384(5):403-16.
Efficacy
The study found that the vaccine was 94.1% effective (95% confidence interval 89.3-96.8) in the prevention of symptomatic SARS-CoV-2 infection as compared to the placebo. Upon subgroup analysis, including age groups, sex, race, and ethnic groups, the efficacy of the vaccine was consistent across all groups. Between day 1 and day 42, seven cases of COVID-19 were identified in the vaccine group, compared to 65 cases in the placebo group.
Local Effects
The most common local injection site event was pain. Local events lasted and resolved themselves over a 2–3-day period. Delayed reactions occurred 8 days post injection, and included erythema, induration, or tenderness. This delay occurred in 0.8% of participants following their first dose and 0.2% of participants after their second injection, resolving over 4-5 days. Erythema, swelling and local lymphadenopathy occurred in both the placebo and vaccine group, but more commonly in the vaccine group following the second injection.
Systemic Effects
The presence of systemic effects occurred both in the placebo and vaccine group, although occurred more frequently in the vaccine group following the first and second doses. Severity of systemic effects were increased after the second dose compared to the first and were more commonly reported by the younger population (18-65 years-old).
Adverse Events
Hypersensitivity reactions to both the vaccine and placebo occurred at a rate of 1.5% and 1.1%, respectively. Bell’s palsy was observed in three vaccine participants and one placebo participant in the observation period, more than 28 days after injections. Overall, adverse events that were thought to have transpired due to either the placebo or vaccine was 4.5% and 8.2% of the participants, respectively. The most common treatment-related adverse event in both the placebo and vaccine group was fatigue and headache. The incidence of treatment-related severe adverse events was higher in the vaccine group. There was no evidence of vaccine-associated respiratory disease, and fewer cases of both severe and non-severe COVID-19 occurred in the vaccine group versus the placebo group. Three deaths occurred in the placebo group – one from intra-abdominal perforation, one from cardiopulmonary arrest and one from severe systemic inflammatory syndrome in a patient with Chronic Lymphoblastic Leukaemia. Two participants in the vaccine group – one from cardiopulmonary arrest and one from suicide.

Research Summary figure reprinted with permission from Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. New England Journal of Medicine. 2020;384(5):403-16.
Authored by Maria Casalino. Edited by Dr. Ahuva Brown.
September 2, 2020
Premature Births and Stillbirths
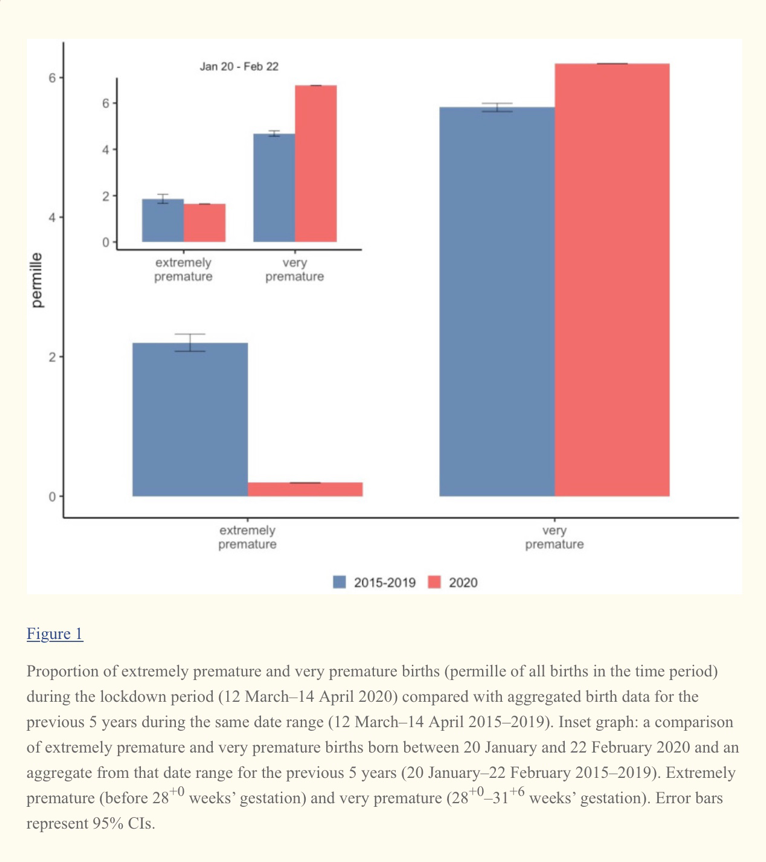
Article 1: Danish Premature Birth Rates During the COVID-19 Lockdown
- Lower rate of extremely premature children during the lockdown when compared to the corresponding mean rate for the same dates in the previous 5 years
- No significant difference was found between the lockdown period and gestational age categories
- Elements of the lockdown (reduced physical activity, for example) could be beneficial for reducing extreme prematurity
Article 2: Change in the Incidence of Stillbirth and Preterm Delivery During the COVID-19 Pandemic
- Note: this research was performed in the UK
- The incidence of stillbirths was higher in the pandemic period than the pre-pandemic period (none related to COVID-19 infections)
- None of the stillbirths during the pandemic, however, were born to women with confirmed COVID-19
- Increase in stillbirths may related to indirect effects of the pandemic such as reluctance to going to hospital when needed (ex. Reduced fetal movements) or fear of contracting infection
March 26, 2020
What We Know
As novel coronavirus (SARS-CoV-2/ COVID-19) continues to sweep across the world, the literature describing the paediatric presentation of this pandemic remains scarce. In Wuhan, China patients < 1 year of age had the highest percentage of critical cases in the pediatric population1. Therefore, it is imperative to reduce the risk of transmission to this vulnerable group and remain up-to-date on the current guidelines.
Presentation:
While the spectrum of clinical manifestations has yet to be fully described, adults and children both typically present with varying degrees of respiratory illness and gastrointestinal upset. The majority of pediatric cases present mild, with potential symptoms including cough, nasal congestion, rhinorrhea, and sore throat2.
Vertical Transmission:
There are no current reports of vertical transmission of COVID-193. In Huijun, Chen et al investigated the potential intrauterine vertical transmission of COVID-19. Samples of the amniotic fluid, cord blood, neonatal throat swab and breastmilk were taken at the time of delivery of six women with confirmed COVID-191. All tests remained negative for COVID-19, suggesting intra-uterine transmission of the virus does not occur1. Although, some have suggested that temporarily separating the mother and baby after birth (for example, room separation) could aid to further reduce the risk of transmission4.
Breastfeeding:
The World Health Organization (WHO) currently states that it is safe for mothers with COVID-19 to breastfeed as long as they practice the necessary precautions5 which include wearing a surgical mask, washing their hands and breast before and after they breastfeed, as well as thoroughly cleaning surfaces and objects with which they have come into contact5. The American College of Obstetricians and Gynaecologists adds that if a woman is using a manual or electric breast pump, proper cleaning measures of both the pump and the mothers hands must be followed and encourages having someone who is well feed the baby4.
References:
1. Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. The Lancet. 2020;395(10226):809-815.
2. Coronavirus Disease 2019 (COVID-19) [Internet]. Centers for Disease Control and Prevention. 2020 [cited 24 March 2020]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/pediatric-hcp.html
3. Cruz A, Zeichner S. COVID-19 in children: initial characterization of the pediatric disease. Pediatrics. 2020; doi: 10.1542/peds.2020-0834
4. Novel Coronavirus 2019 (COVID-19) [Internet]. Acog.org. 2020 [cited 24 March 2020]. Available from: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/novel-coronavirus-2019
5. WHO, Department of Communications, Maternal, Newborn, Child and Adolescent Health, and Ageing. Q and A on COVID-19 https://www.who.int/news-room/q-a-detail/q-a-on-covid-19-pregnancy-childbirth-and-breastfeeding Accessed March 2020.
Authored by Maria Casalino. Edited by Dr. Elizabeth Mancuso.
Recommendations
Paediatric COVID-19 Literature Review Update
Neonatal Resuscitation and Postresuscitation Care of Infants Born to Mothers with Suspected or Confirmed SARS-CoV-2 Infection
This article discussed the following key points:
Delivery of a newborn to a COVID-19 Positive Mother
-
- NY State currently allows one support person present during the time of delivery – they must be asymptomatic with a negative COVID-19 test
- There is not enough current evidence to support or rebut antenatal steroids in COVID-19 positive mothers awaiting preterm delivery
- WHO recommends that “mothers and infants with suspected or confirmed COVID-19 have the option to remain together with skin-skin contact, especially following delivery”
- Immediate cord clamping should be performed on mothers with suspected or confirmed COVID-19
- 20-60 minutes before delivery of a newborn to a COVID-19 positive mother, the neonatology team should be informed
- The neonatology teams should be prepared with a PPE grab and go kit
Vertical Transmission & Breastfeeding
-
- No current cases that support vertical transmission
- Recent reports show elevated COVID-19 IgM in newborns born to mothers with confirmed COVID-19 but more evidence needed
- Delayed cord clamping does not increase risk of vertical transmission (obstetric provider should hold baby throughout delayed cord clamping)
- No reports of SARS-COV-2 spread through breastmilk – WHO currently supports continuation of breastfeeding with essential hygienic practices and protocols
- SARS-COV-2 antibodies have been discovered in breastmilk
Neonatal Resuscitation
-
- Neonatal resuscitators should be held in the delivery room
- The neonatal resuscitation team should be accessible outside the delivery room, if needed
- The optimal location for neonatal resuscitation remains unclear – it may take place in the delivery room (must remain 6 feet away from the mother with a physical barrier such as a curtain) or in an alternative room
- Newborn resuscitation protocols should follow AAP and NRP recommended parameters – it is important that the correct PPE be worn at all times
- The most experienced provider should perform intubation and other procedures to limit aerosol generation
Post Resuscitation Care
-
- Post resuscitation, the newborn should be transported in a closed isolette and isolated in a negative pressure room
- Having a specialized team that cares for babies born to COVID-19 positive mothers could aid to minimize spread to other healthcare workers
- Newborns to COVID-19 positive mothers should be tested at or beyond 24 hours
- A chest X-ray is indicated in COVID-19 positive newborns that display respiratory symptoms
- Chest x-rays demonstrate signs of pneumonia
- Typical Lab analysis shows leukocytosis, lymphopenia, thrombocytopenia and elevated creatinine kinase-MB fraction
- There is no current evidence that demonstrates increased outcomes upon the administration of Immunoglobulins, antivirals or steroids
- A healthy caregiver may care for the newborn until the mother is afebrile (without antipyretics) and has two negative tests for SARS-COV-2 at least 24 hours apart
The possibility of infection from SARS-COV-2 should be considered in the ill infant for the foreseeable future
Click here for the full article: Chandrasekharan, P., Vento, M., Trevisanuto, D., Partridge, E., Underwood, M.A., Wiedeman, J., Katheria, A. and Lakshminrusimha, S., 2020. Neonatal Resuscitation and Postresuscitation Care of Infants Born to Mothers with Suspected or Confirmed SARS-CoV-2 Infection. American Journal of Perinatology.
Clinical and CT features in pediatric patients with COVID‐19 infection: Different points from adults
This article discussed the following key points:
- The most common presenting symptoms were fever and cough
- Procalcitonin (PCT) was elevated in 80% of cases whether or not coinfection existed, which is uncommon in the adult population.
- Majority of patients had a normal WBC count
- On CT, the most common finding was unilateral or bilateral subpleural ground‐glass opacities, and consolidations with surrounding halo sign. “As consolidations with surrounding halo sign account for up to 50% cases, they should be considered as typical signs in pediatric patients.”
- Pleural effusions were not seen on CT
Click here for the full article: Xia, W., Shao, J., Guo, Y., Peng, X., Li, Z. and Hu, D., 2020. Clinical and CT features in pediatric patients with COVID‐19 infection: Different points from adults. Pediatric Pulmonology.
A Well Infant With Coronavirus Disease 2019 With High Viral Load
This is an interesting case study of a 6 month old infant with confirmed SARS COV-2 in Singapore.
Key points:
- The infant was brought in for isolation due to both of his parents having tested positive for SARS COV-2
- Presented asymptomatically, afebrile, with no tachypnoea and an oxygen saturation of 98%
- A nasopharyngeal swab was performed on the infant and he tested positively for SARS COV-2
- He remained asymptomatic throughout the his admission
- His stool tested positive for SARS-COV-2 on admission day 9
- This opens up the possibility of a much higher number of pediatric SARS-COV-2 cases in the community that remain asymptomatic as compared to the reported confirmed cases
Click here for the full article: Kam, K.Q., Yung, C.F., Cui, L., Lin Tzer Pin, R., Mak, T.M., Maiwald, M., Li, J., Chong, C.Y., Nadua, K., Tan, N.W.H. and Thoon, K.C., 2020. A well infant with coronavirus disease 2019 (COVID-19) with high viral load. Clinical Infectious Diseases.
